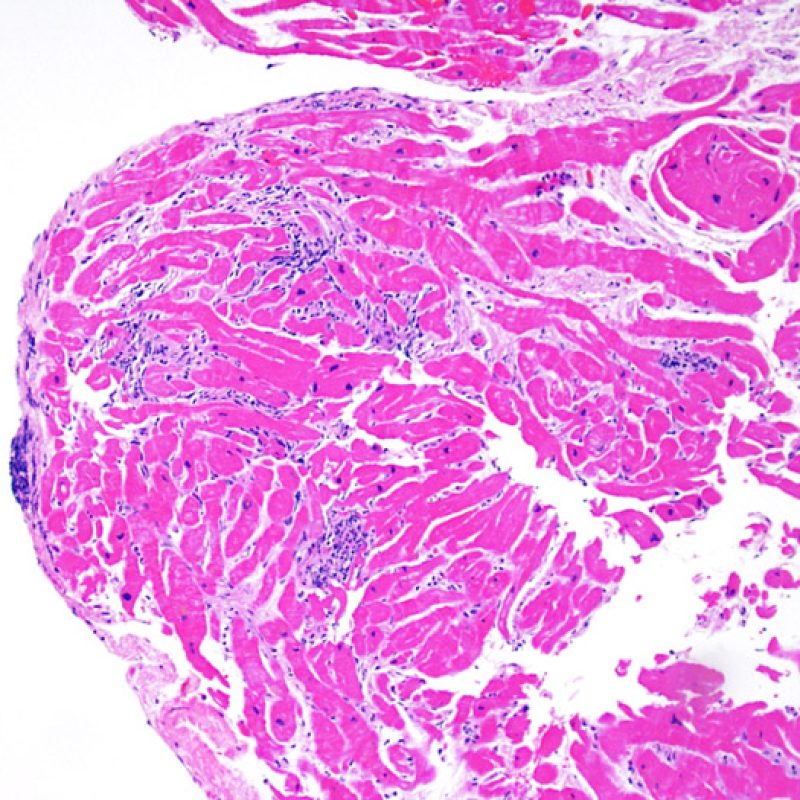
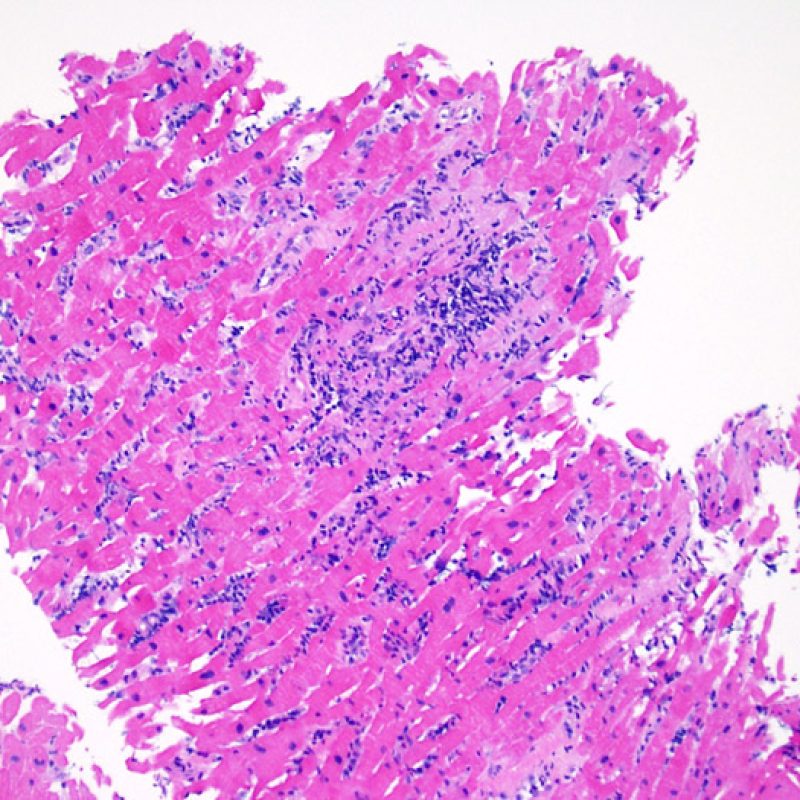
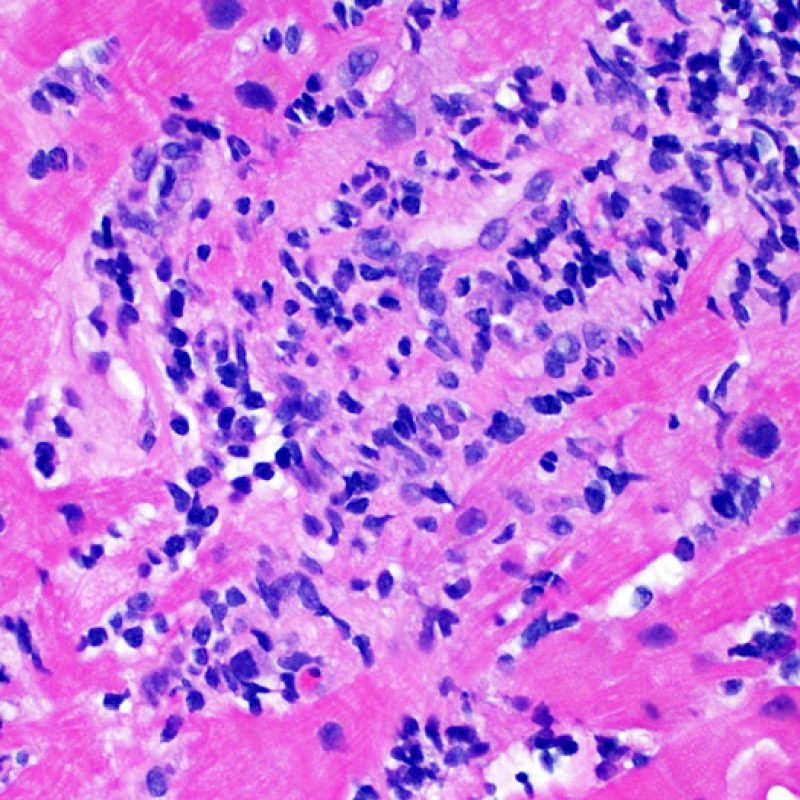
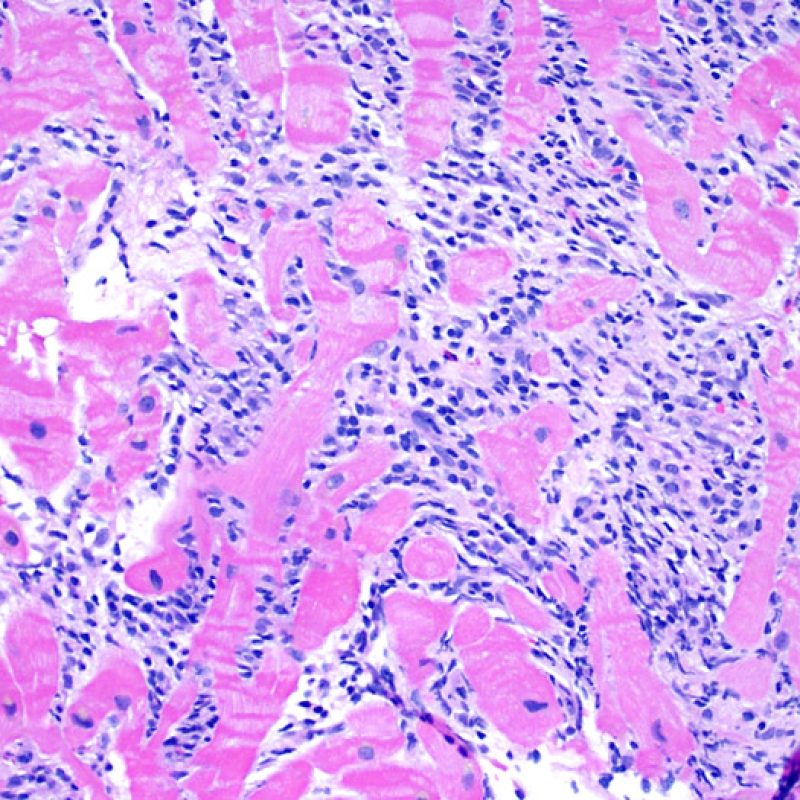
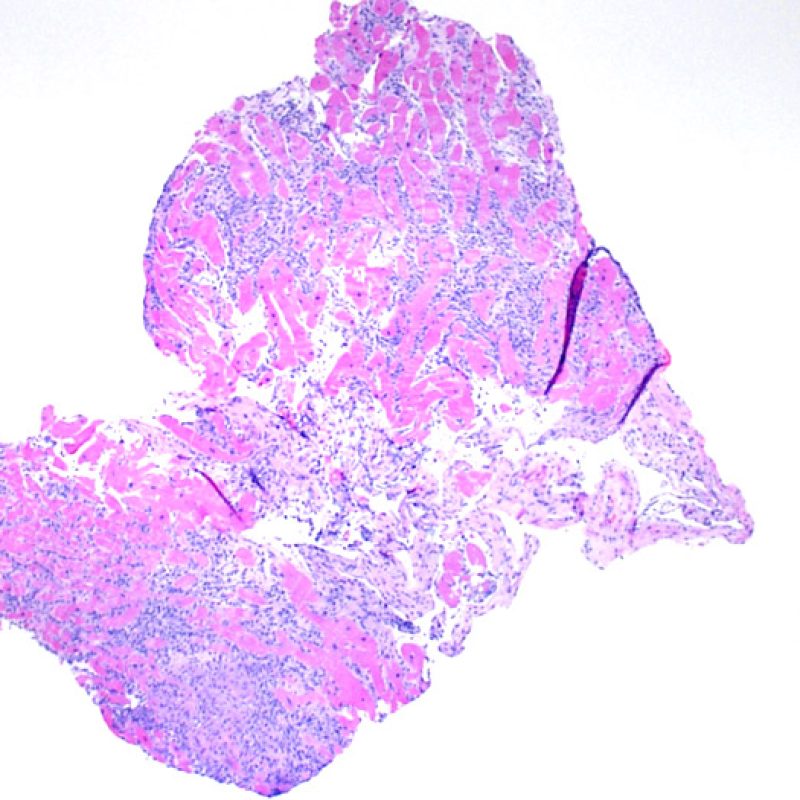
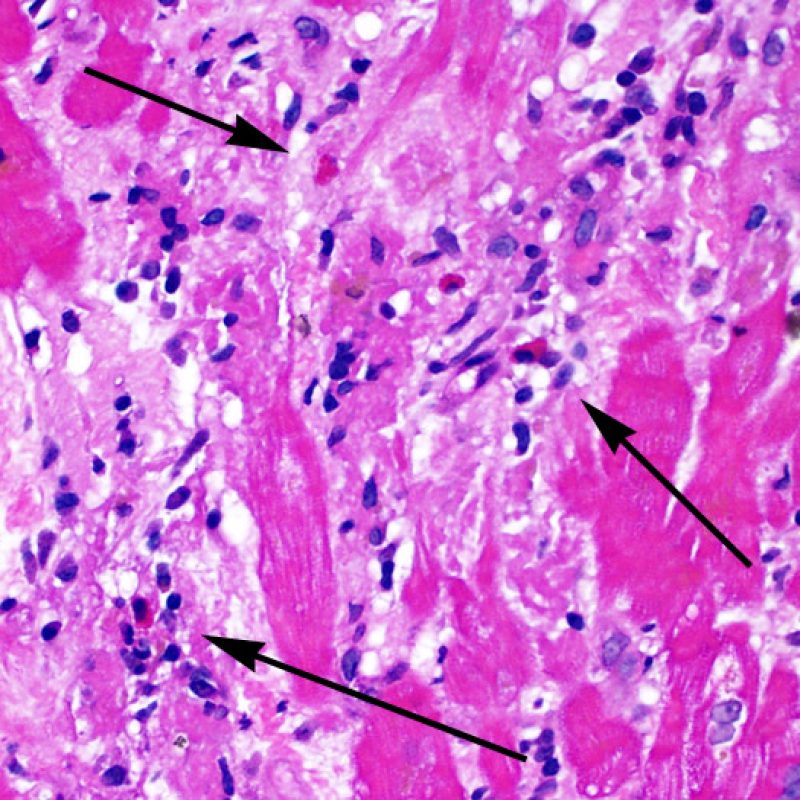
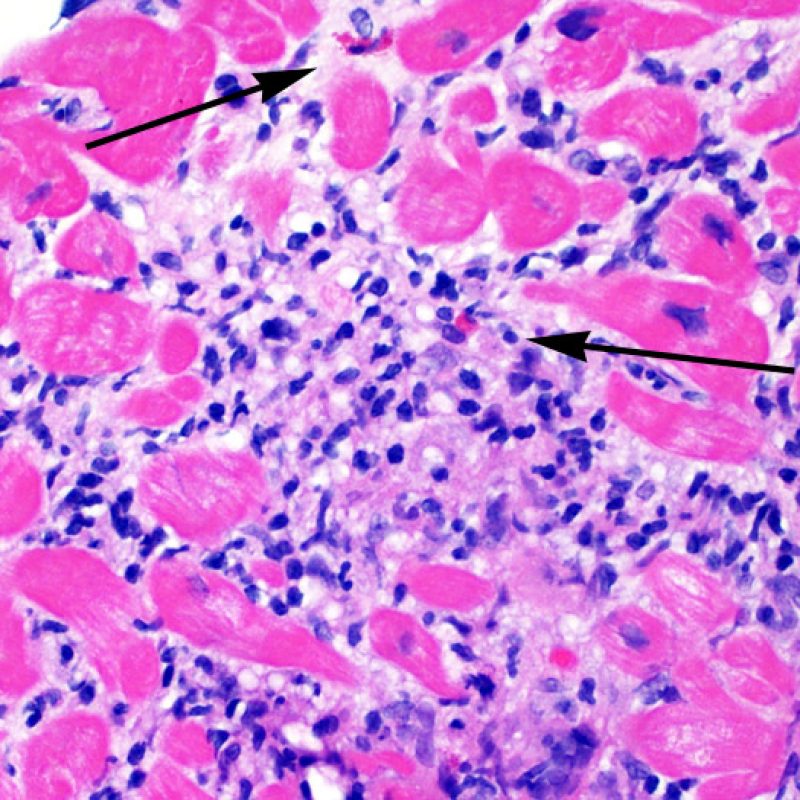
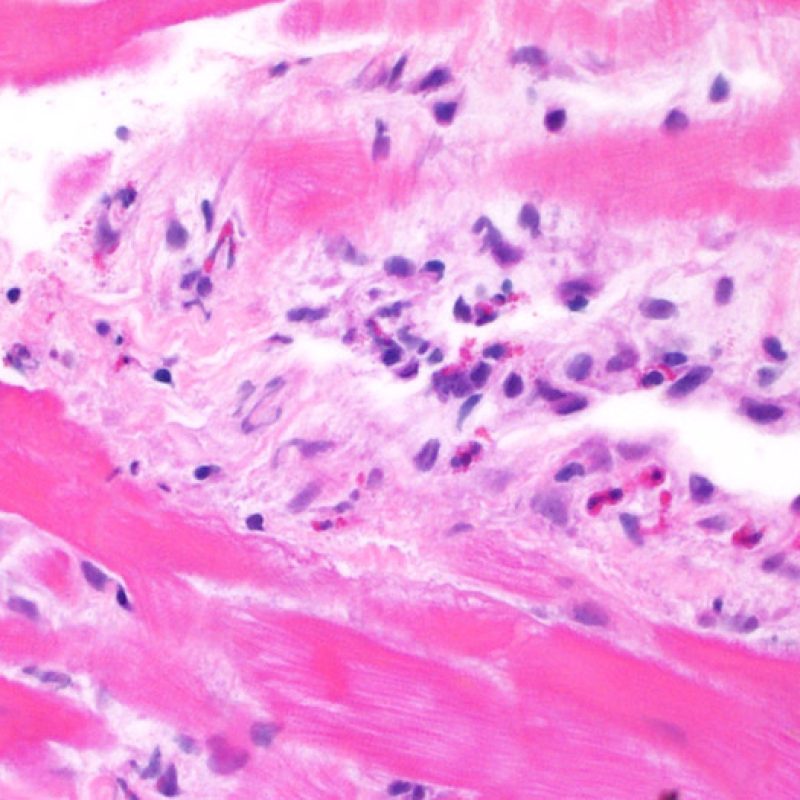
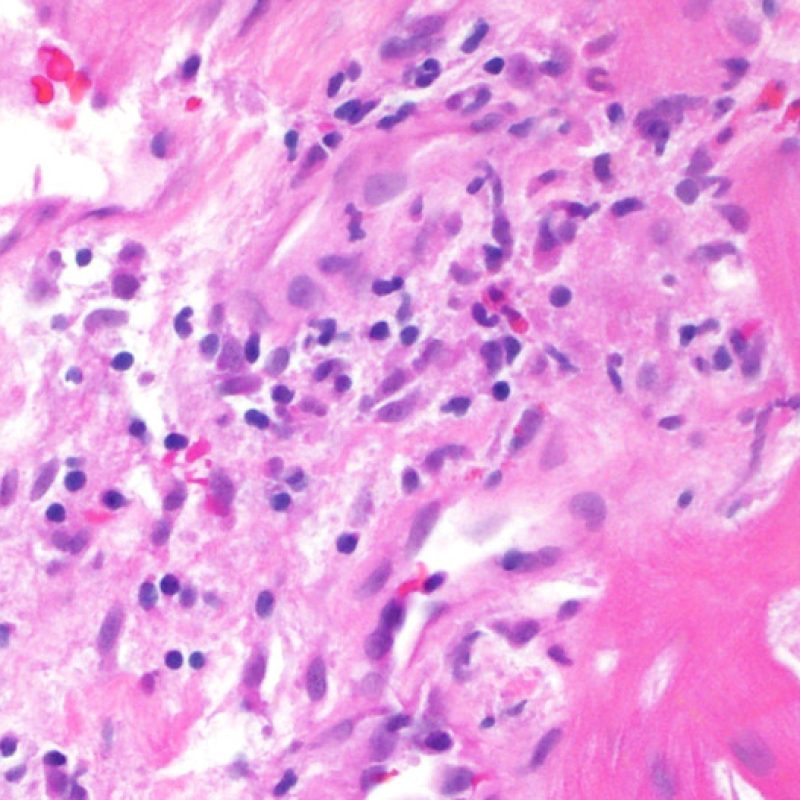
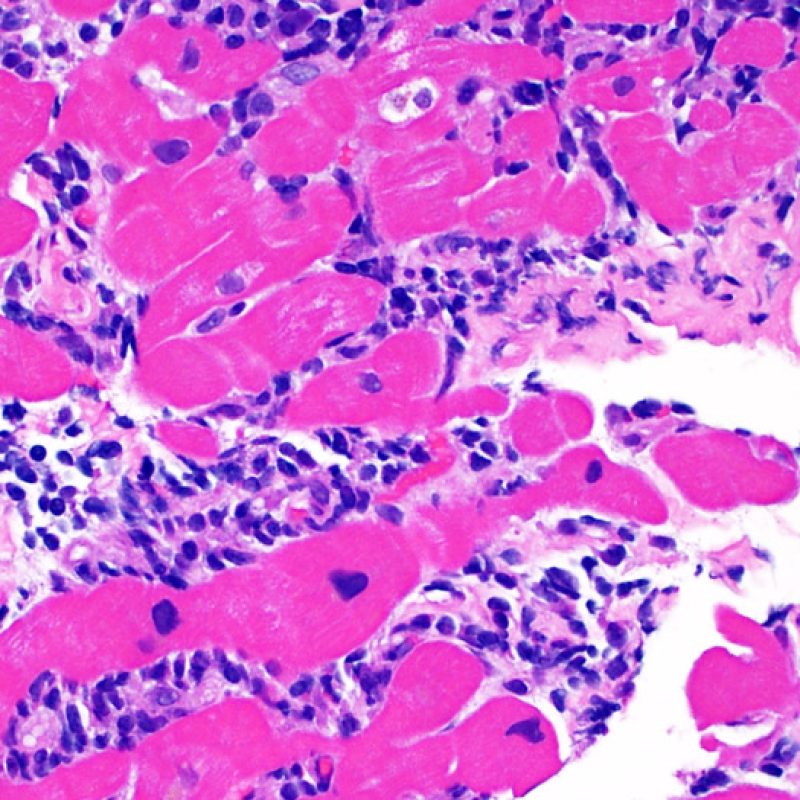
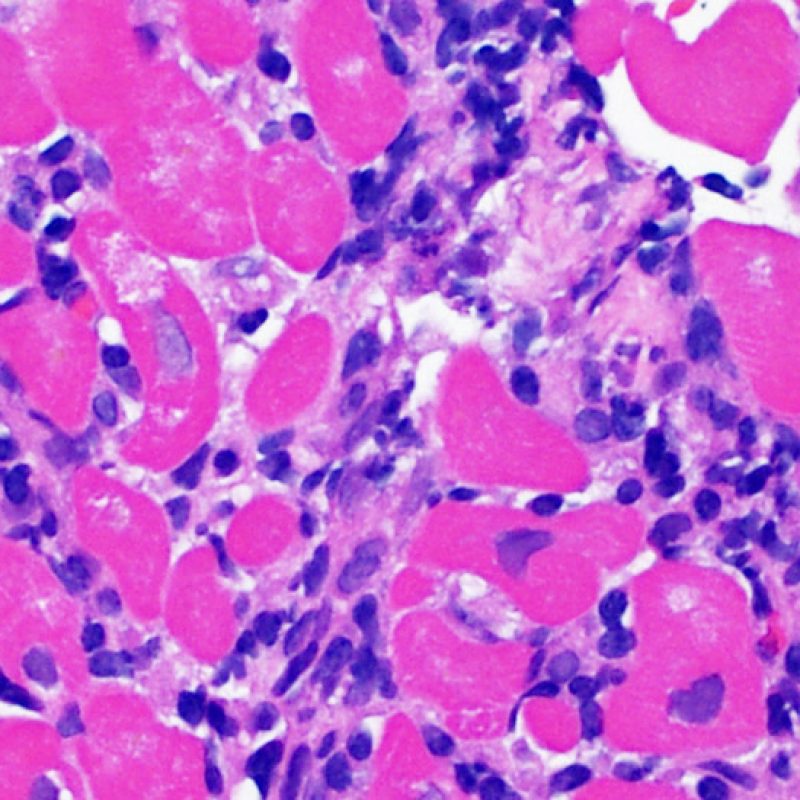
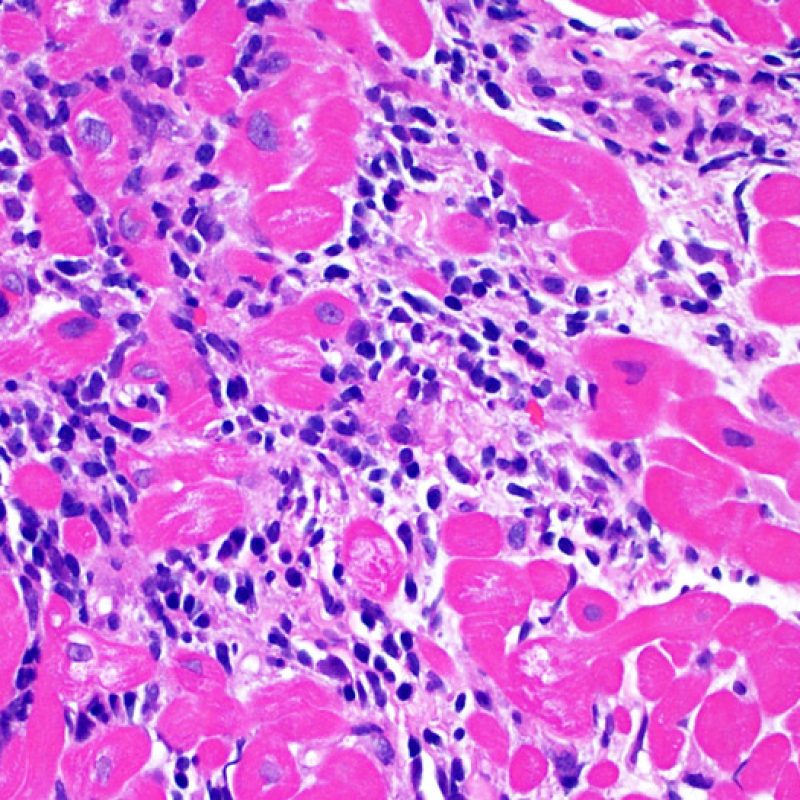
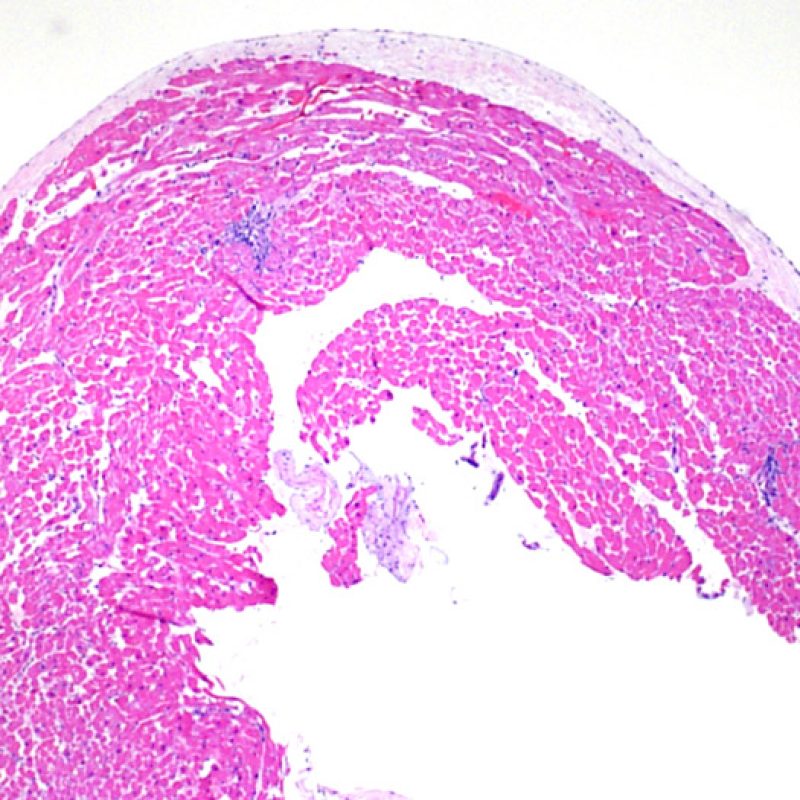
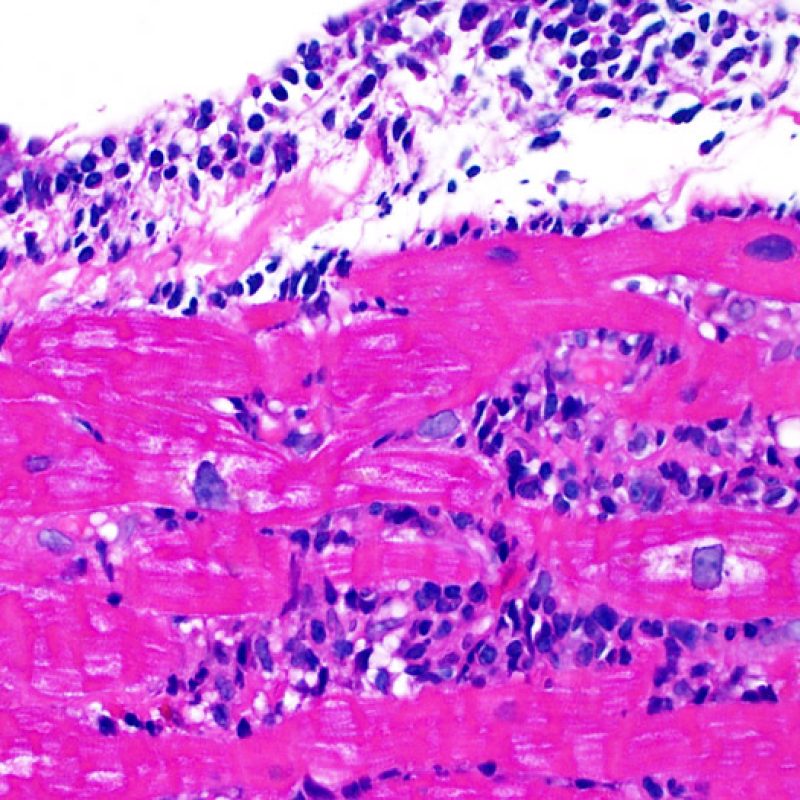
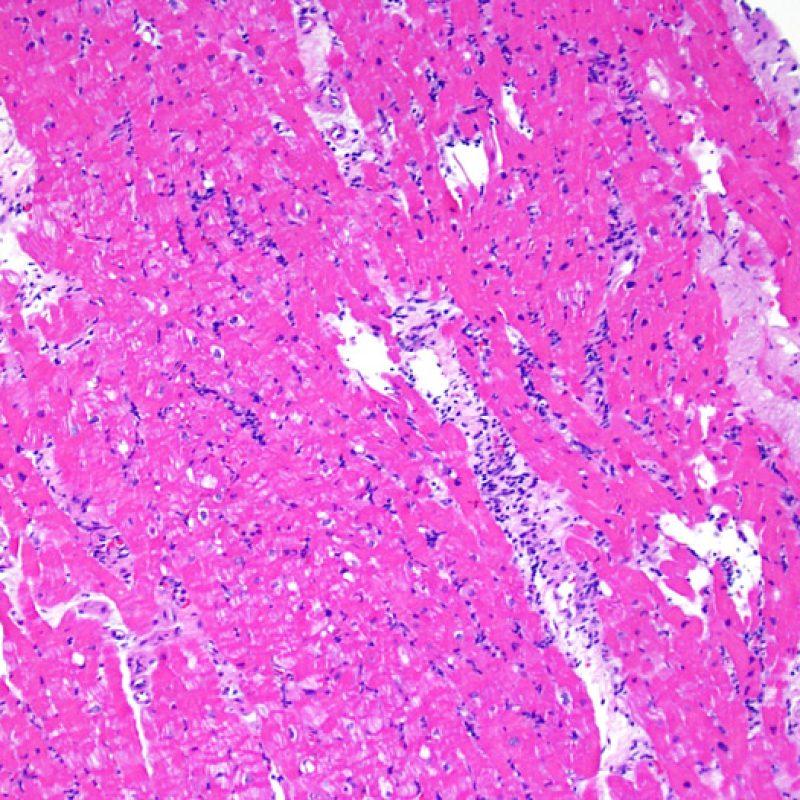
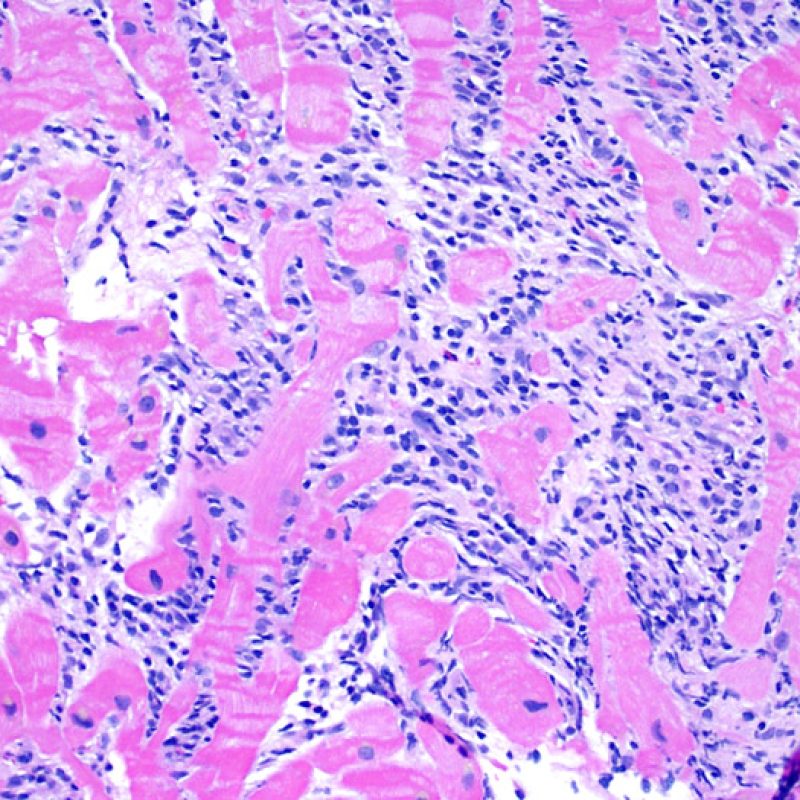
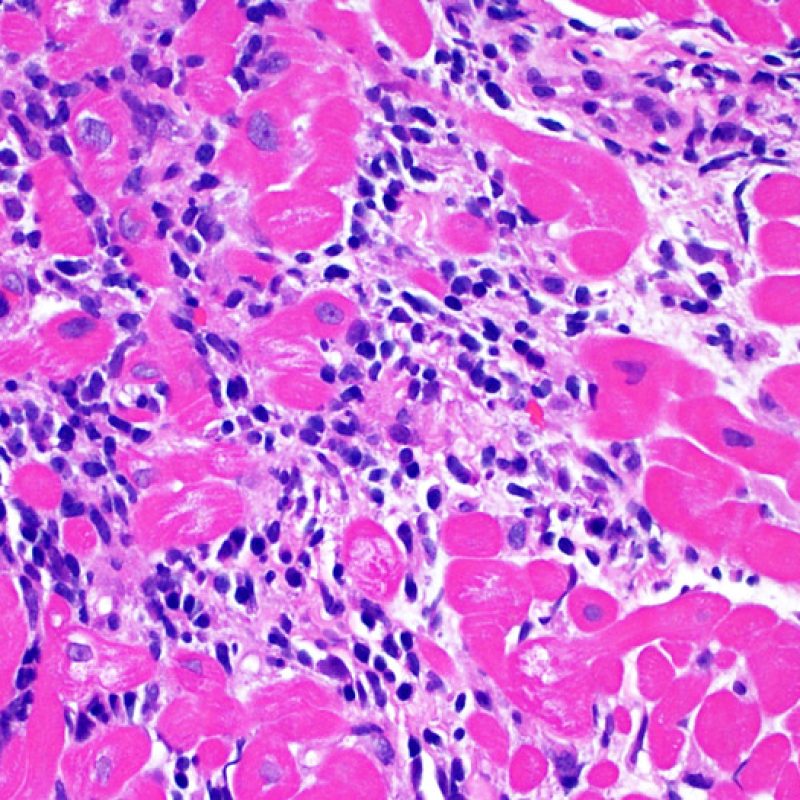
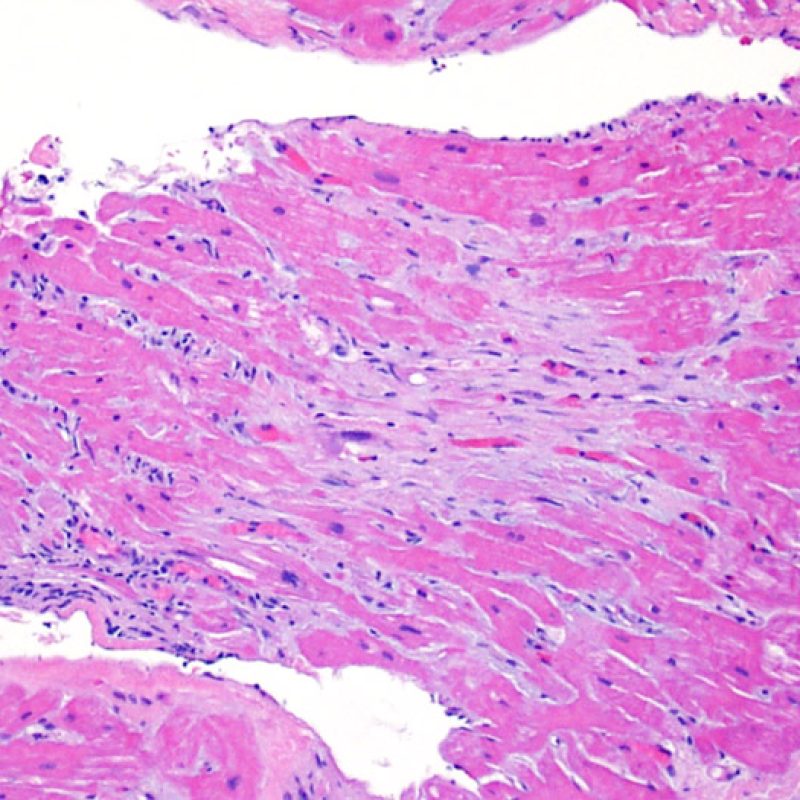
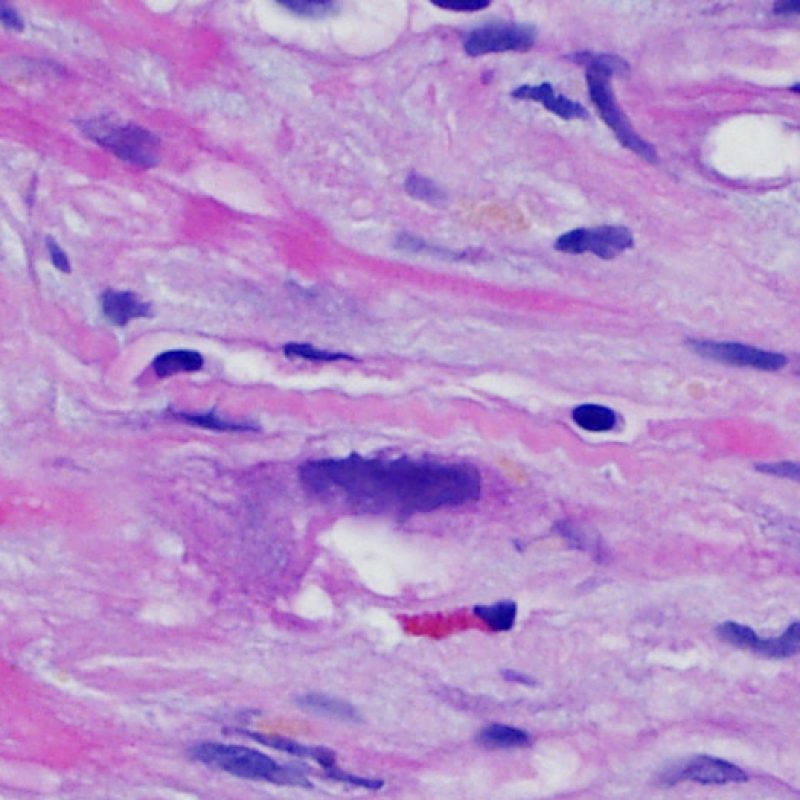
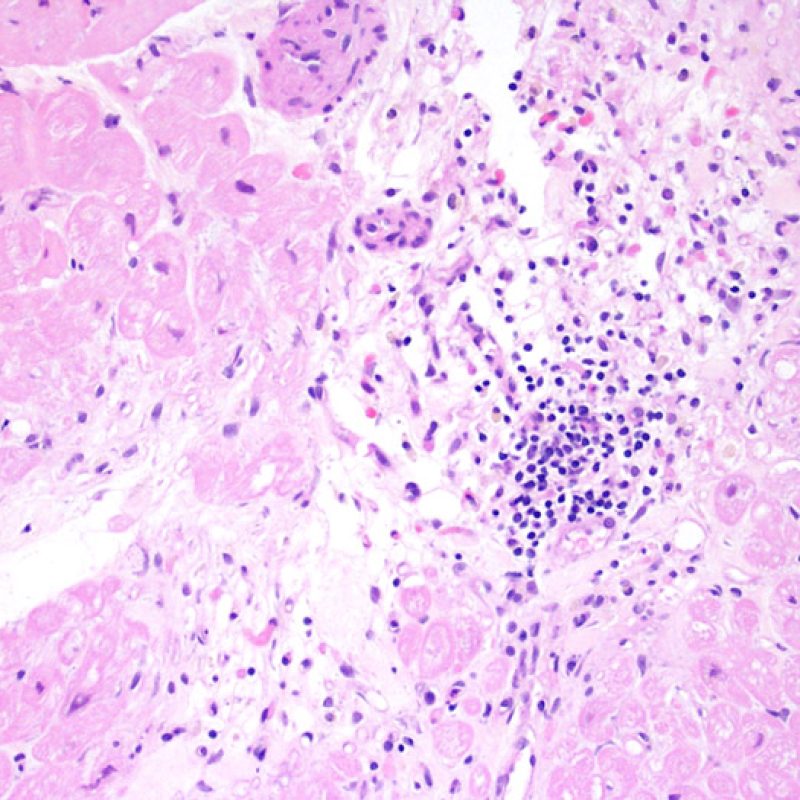
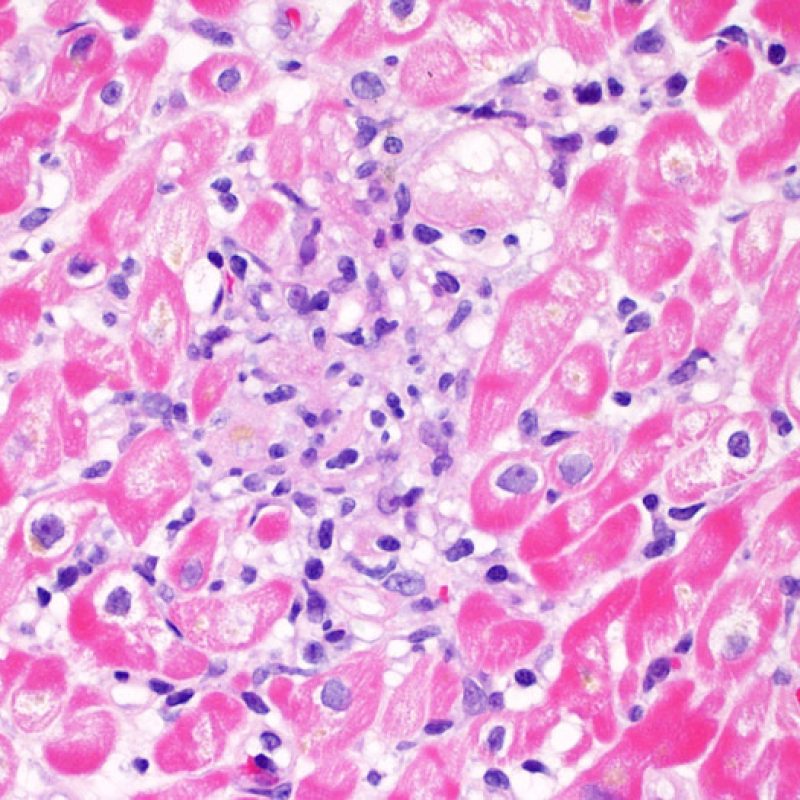
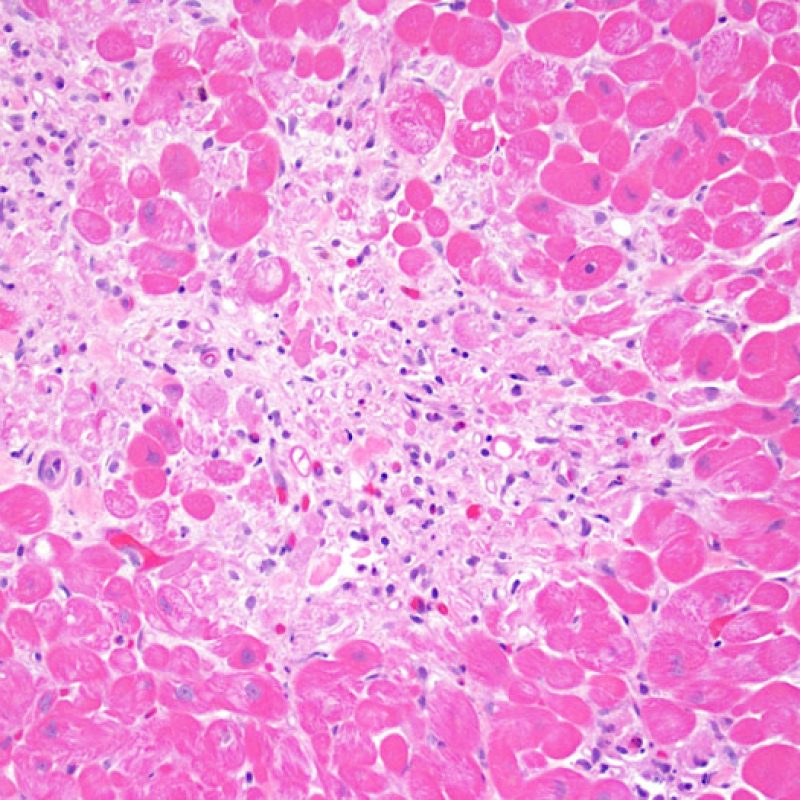
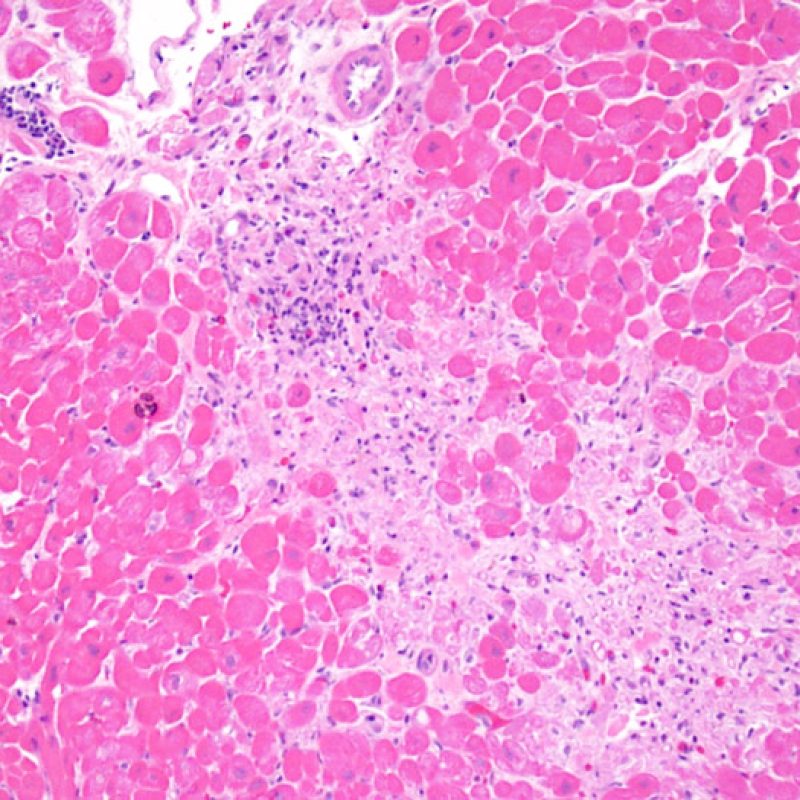
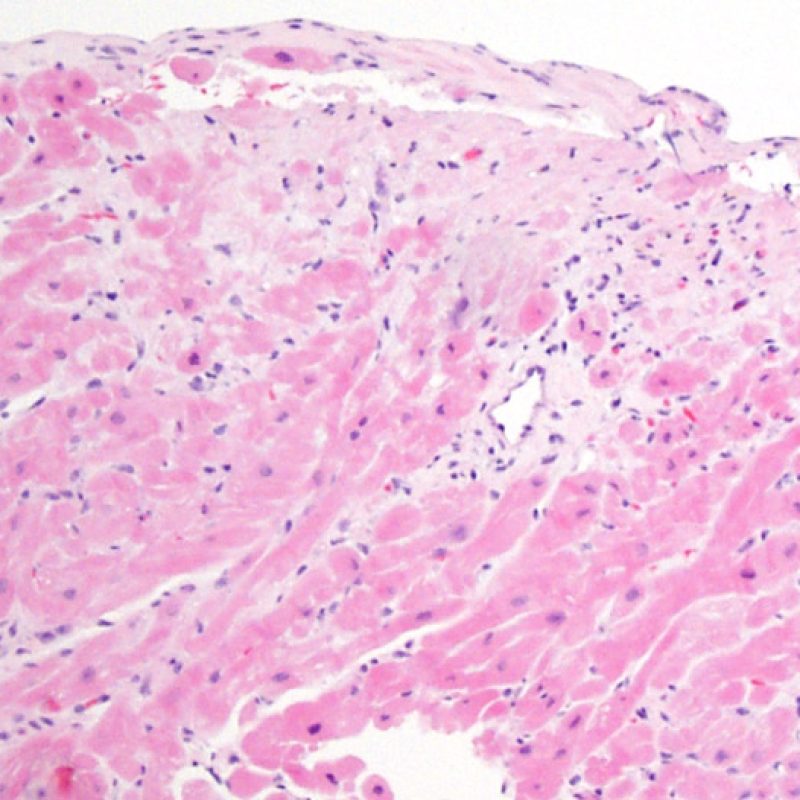
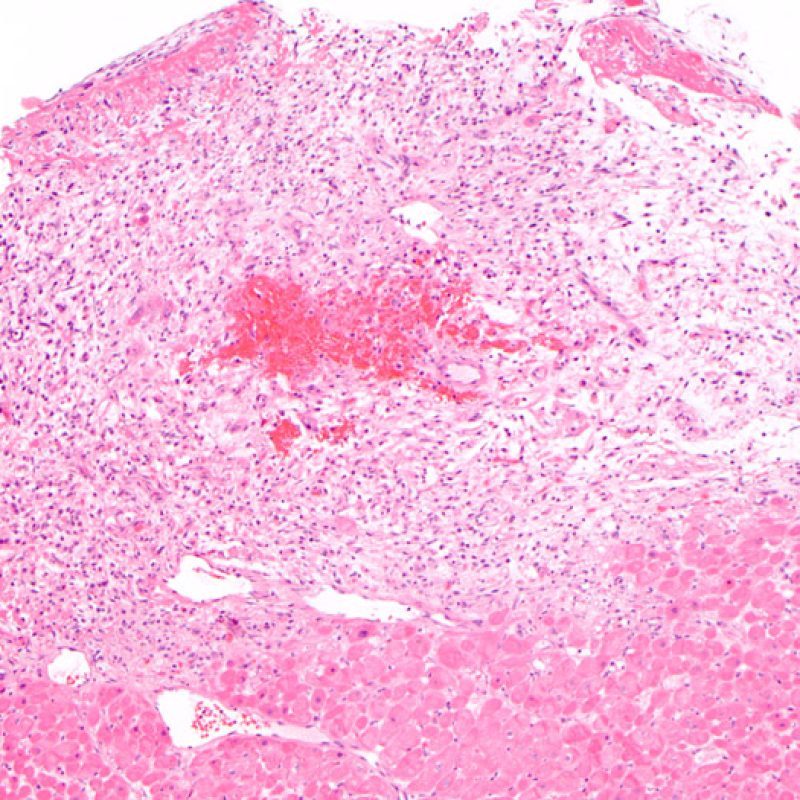
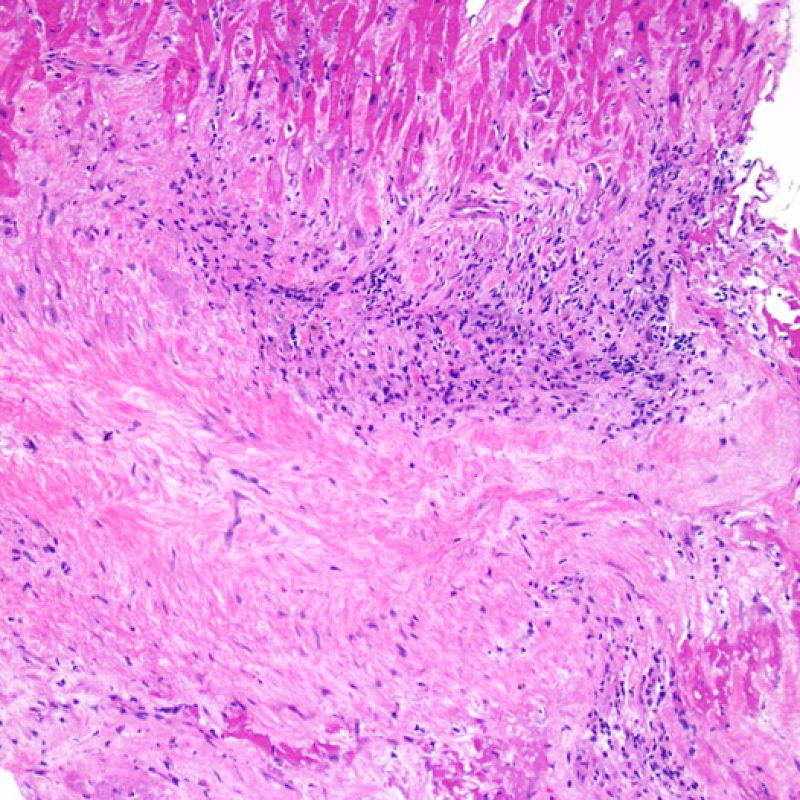
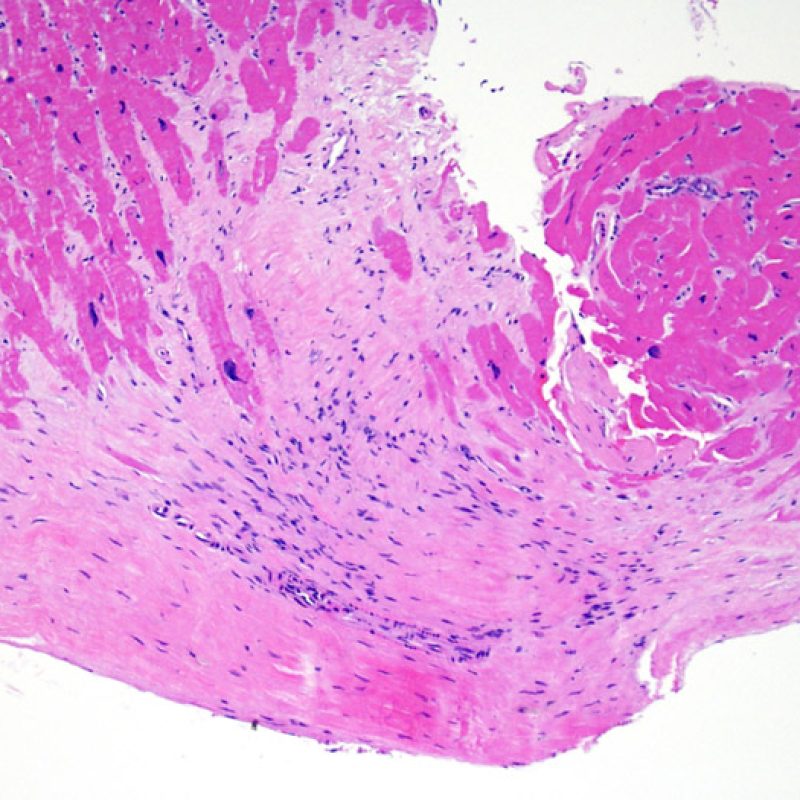
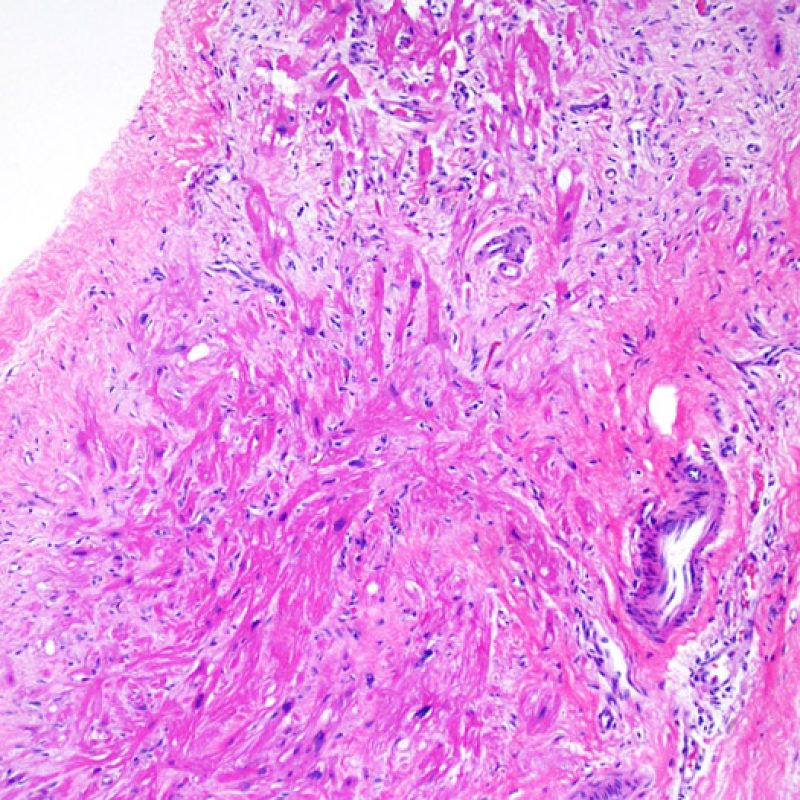
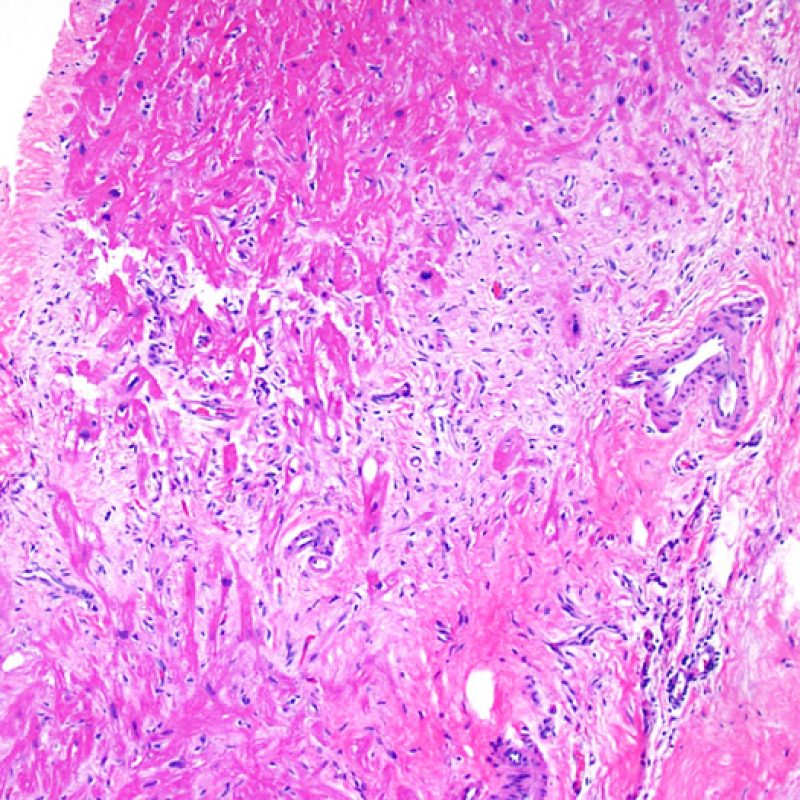
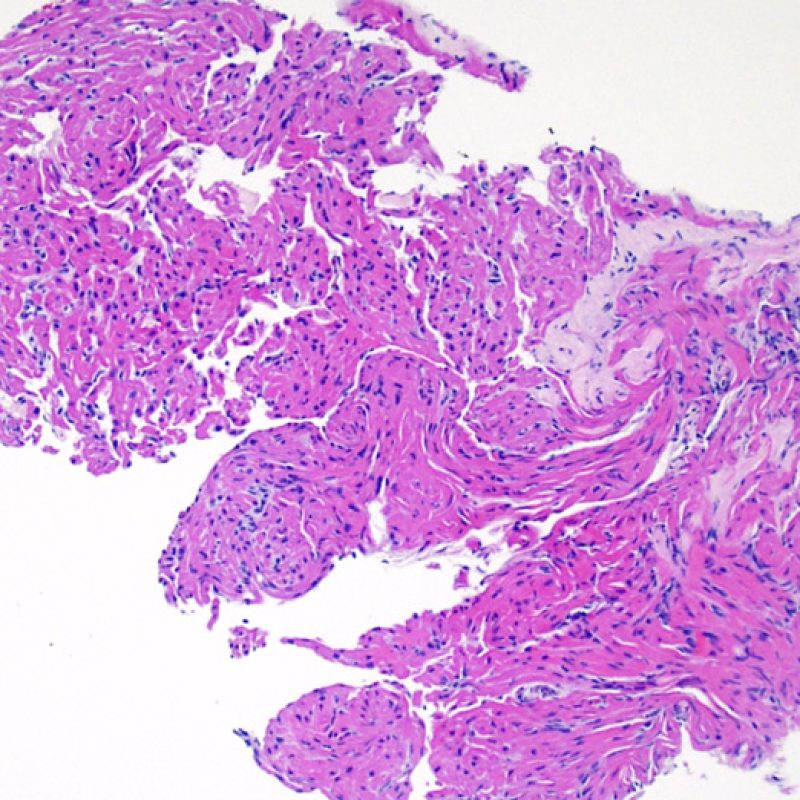
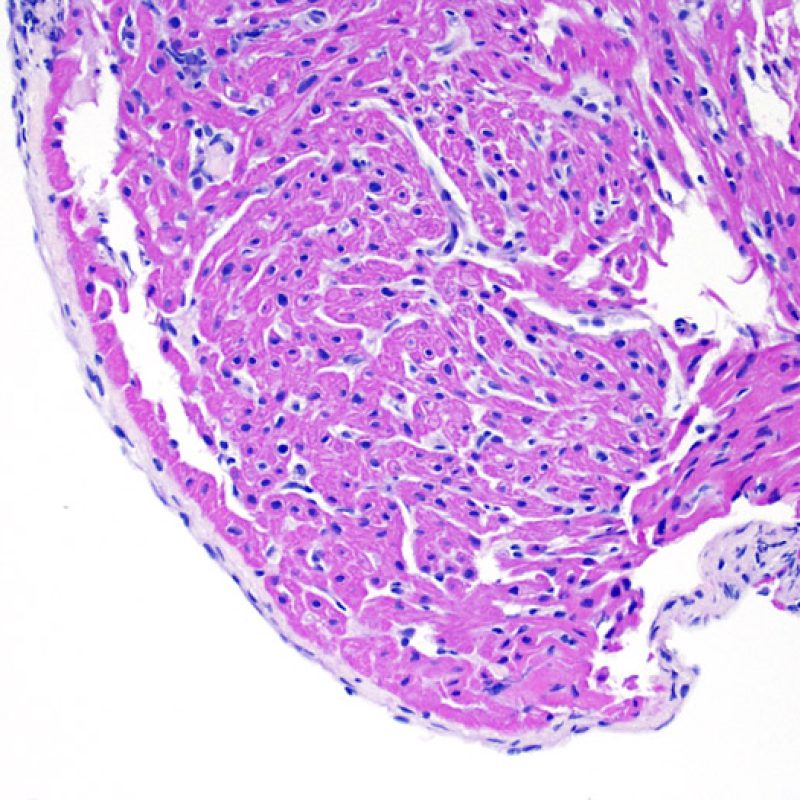
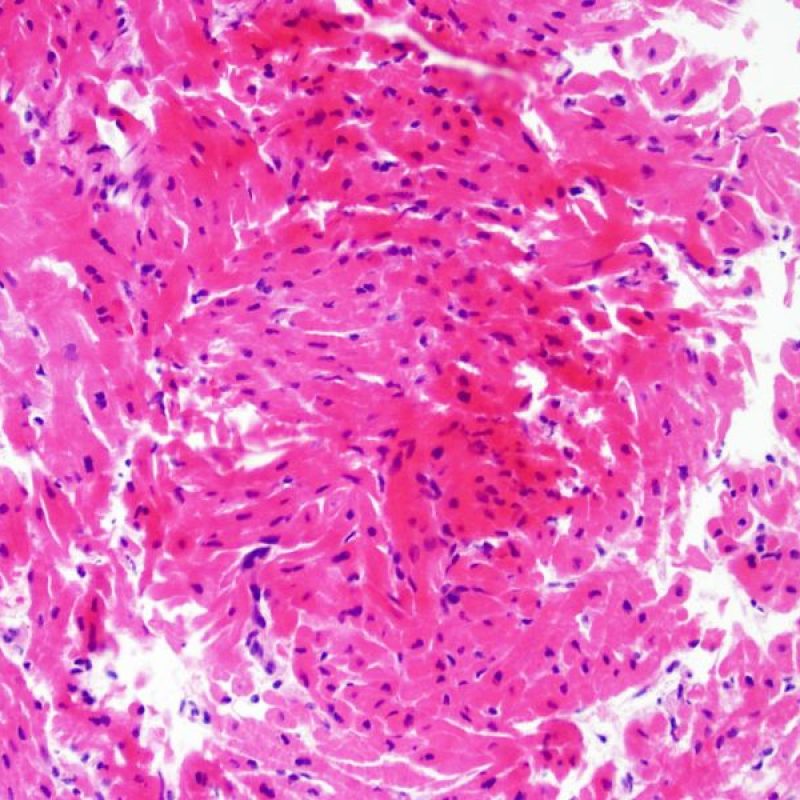
Overview
Welcome to the Society for Cardiovascular Pathology (SCVP) and Association for the European Cardiovascular Pathology (AECVP) web tutorial on interpreting endomyocardial biopsy findings for cardiac transplant acute cellular rejection. The goal of this tutorial is to educate pathologists on a method of interpretation and on the histopathologic findings one sees on this biopsy material.
This tutorial contains 7 sections covering all aspects of the endomyocardial biopsy. The menu on the left hand side is clickable and can allow the user to move directly to an area of interest. All images can be enlarged by clicking on them (click on the picture to shrink it down). A self-testing quiz using virtual slides of actual cases has been created to make sure you are able to determine cellular rejection and not confuse other histologic findings.
This tutorial has gone through a two-step process of document creation and editing. The members of this committee can be found on the about page and they are solely responsible for the material contained herein. A tutorial focusing on antibody-mediated rejection (AMR) can be found here. We have created a short survey to get some feedback on the usage of this web tutorial. Please help us better understand the audience of our tutorial and how it can be improved. This survey is only being administered for a few weeks.
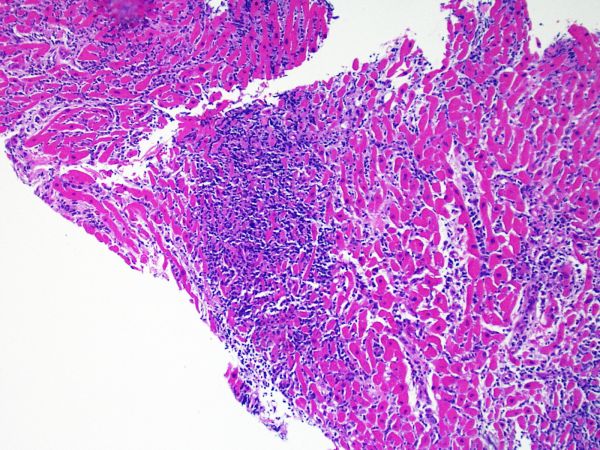
The endomyocardial biopsy
The endomyocardial biopsy (EMB) is an established clinical procedure used for obtaining myocardial tissue. It is typically performed under local anesthesia by passing a bioptome (schematic) through the right internal jugular vein across the tricuspid valve and into the right ventricle. Sampling is usually performed under fluoroscopic (or less commonly echocardiographic) guidance along the interventricular septum.
The most common and established indication for EMB is surveillance of cardiac transplant rejection status, including both cell-mediated and antibody-mediated types. It remains the only method to reliably diagnose cardiac rejection. The surveillance generally results in numerous biopsies, according to each transplant center’s established protocol, especially within the first few months of transplant, which then trail off based on the individual style of the institution.
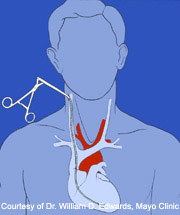
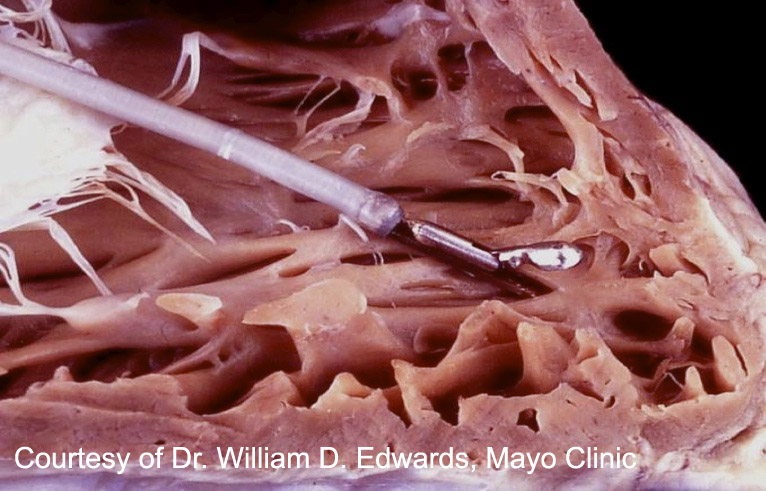

The endomyocardial biopsyHow to process a biopsy
Number of samples to obtain: The International Society for Heart and Lung Transplantation (ISHLT) recommendations are to obtain a minimum of 3 right ventricular samples to appropriately assess for transplant rejection; however increasing to 5 samples has been shown to improve sensitivity. Of note, these samples should be independently collected and not divided after procurement to increase the sample size.
Manipulation of tissue: The cardiolgy team should remove the specimen off the biopsy catheter with a needle and place it on gauze soaked in isotonic saline. The specimen should be minimally handled after procurement and should not be manipulated with forceps.
Processing of tissues: For assessing transplant rejection, the biopsy material should be immediately stored in 10% neutral buffered formalin. The specimen can then be embedded in paraffin, sectioned and stained according to established laboratory protocols. Depending on institution-specific laboratory protocols, immunofluorescence studies may be used to help monitor for antibody-mediated rejection. One additional sample should be snap frozen if immunofluorescence is planned.
Slides to Process: Most institutions generate 3 or 4 slides containing at least three 3-5um sections stained with H&E. Some institutions put up to 8 sections on a single slide. The important point is that to adequately evaluate the rejection process, a minimum of 10 sections should be viewed, on however many slides an institution chooses to use.
Ancillary studies: C4d immunofluorescence or immunohistochemistry is frequently ordered concurrently with the initial H&E sections to help assess the biopsy sample for changes of antibody-mediated rejection. Some institutions routinely use CD68 (a histiocyte marker) or C3d as part of their investigation of AMR. AMR will be covered extensively in a separate tutorial.
Assessing adequacy
An adequate sample should consist of at least 3 fragments, containing at least 50% myocardium. If insufficient tissue appears to be present, assessing the block to ensure that it was adequately effaced is an important first step. Patients being assessed for rejection often have multiple biopsies. For mechanical reasons the bioptome tends to sample the same region of the ventricle. Therefore it is very likely to sample areas with fibrosis, fat infiltration etc. and it is quite common to see granulation tissue or scar formation from a previously sampled biopsy site. Cardiologists who have performed numerous biopsies become familiar with the color and texture of the biopsies and can differentiate myocardium from thrombus or scar.
What to do with an inadequate biopsy:
- Assess the specimens for rejection, if cell- or antibody-mediated rejection is present, it should be reported regardless of the sample size.
- If the specimen does not meet criteria for adequacy, and shows no rejection, a comment can be made that describes the inadequacy and the possibility of a false-negative result.

Conveying results
The pathology report should be concise and standardized to efficiently convey the relevant information to the patient’s care provider (typically the treating cardiologist). In addition to the presence or absence of rejection, a complete diagnosis can include the number of specimens evaluated (as well as the number involved by rejection), the presence of Quilty lesions, as well as indicating whether features of infection or ischemia were present.
An example template for formatting of pathology reports is provided here.
Grading Criteria
This section of the tutorial will explain the International Society for Heart and Lung Transplantation (ISHLT) revised grading criteria.
Overview of Grading Scheme
In 1990, the International Society for Heart and Lung Transplantation (ISHLT) developed a standardized grading method to determine acute cellular rejection on endomyocardial biopsy. These grading criteria were revised in 2004 and are sometimes refered to as ISHLT-2004. These criteria have 4 levels of rejection (0R, 1R, 2R & 3R). The “R” stands for revised so as not to be confused with the ISHLT-1990 criteria (0, 1A, 1B, 2, 3A, 3B, & 4). At most institutions, a grade of 0R or 1R is not further treated and a grade of 2R or 3R is treated with increased immunosuppression. Thus it is vital for a pathologist to distinguish between low grade (0R & 1R) and high grade (2R & 3R) lesions.
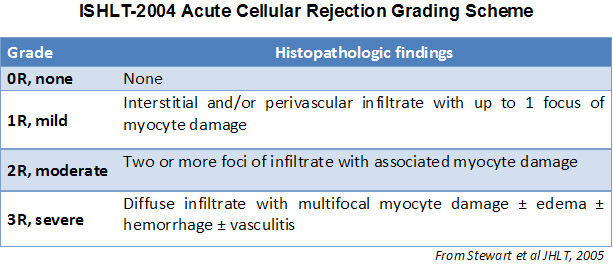
The two key elements of acute cellular rejection are the presence of lymphocytes and myocyte injury. Other features of the endomyocardial biopsy may represent rejection mimickers, artifacts, helpful clues of rejection or the presence of other diseases. Thus it is important to recognize that successful interpretation of an endomyocardial biopsy for rejection is dependent on assessing only two features – infiltration and injury.
No evidence of rejection (OR)
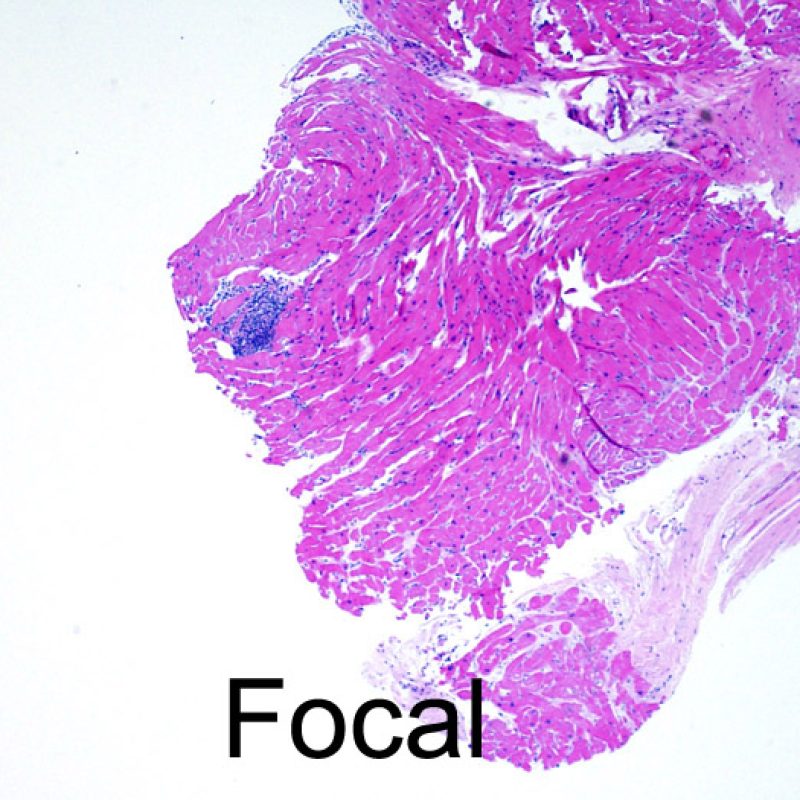
The absence of any lymphocytic infiltration is termed – no evidence of cellular rejection – grade 0R. Essentially this is a clean biopsy containing only unremarkable endomyocardium. There may be non-rejection features on one of these biopsies such as a Quilty effect or biopsy site changes, but these can be reported separately and should not cause an increase in rejection grade.
Mild rejection (1R)
Mild rejection (1R) is defined as interstitial and/or perivascular infiltrate with up to 1 focus of myocyte injury. Because this definition includes any amount of lymphocytes in any pattern, a large and diverse range of biopsies will fit into this expansive category. The infiltrate can range from just a few perivascular lymphocytes to an extensive infiltrate of lymphocytes that are not injurious to more than a single focus of myocytes. The extent of infiltrate can overlap with the extent of infiltrate of a 2R lesion if the infiltrate has not caused more than a single focus of myocyte injury. Generally, eosinophils or neutrophils are not seen in biopsies graded 1R. Biopsies with a single focus of myocyte injury are particularly challenging. Was only a single focus found because the biopsy was barely adequate? Perhaps a Quilty effect is mimicking myocyte injury? It is often useful to convey to the clinician when a biopsy that meets grade 1R criteria is particulary busy or has a single focus of myocyte injury.
Moderate rejection (2R)
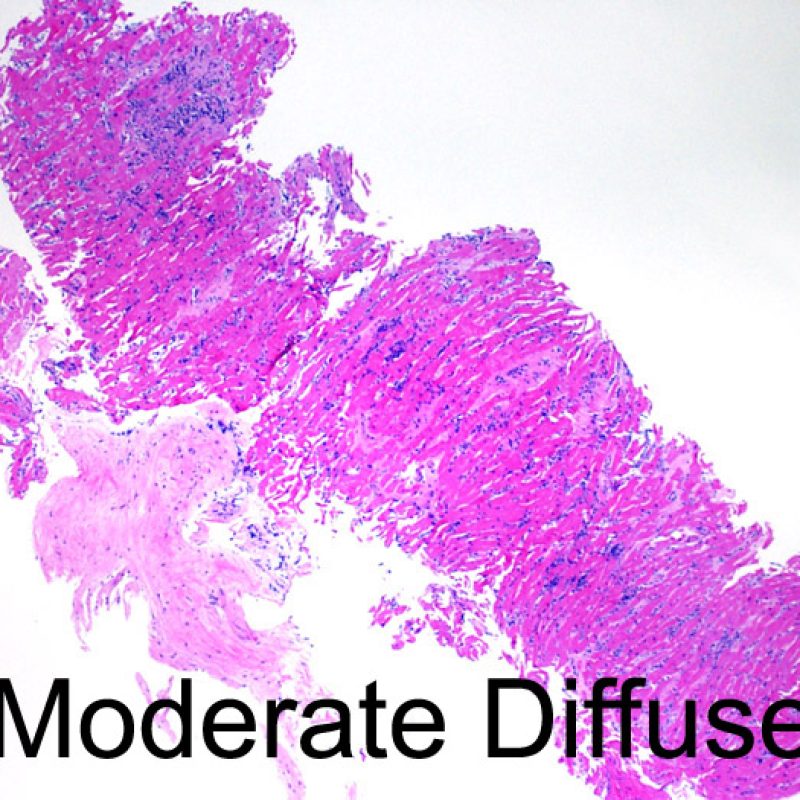
Moderate rejection (2R) is used when there are two or more foci of infiltrate with associated myocyte injury. These foci can be found on the same biopsy piece, separated by a small distance, or they can occur on different pieces or even at different levels through the material. Generally, two or more foci of myocyte injury are associated with at least a moderate cellular infiltrate across the entire biopsy. In the absence of a diffuse infiltrate, one should question whether or not they are overcalling myocyte injury and may think of other mimickers of rejection. It is always a good idea to convey to the clinicians the presence of 2R rejection on a biopsy as immediate treatment is warranted.
Severe rejection (3R)
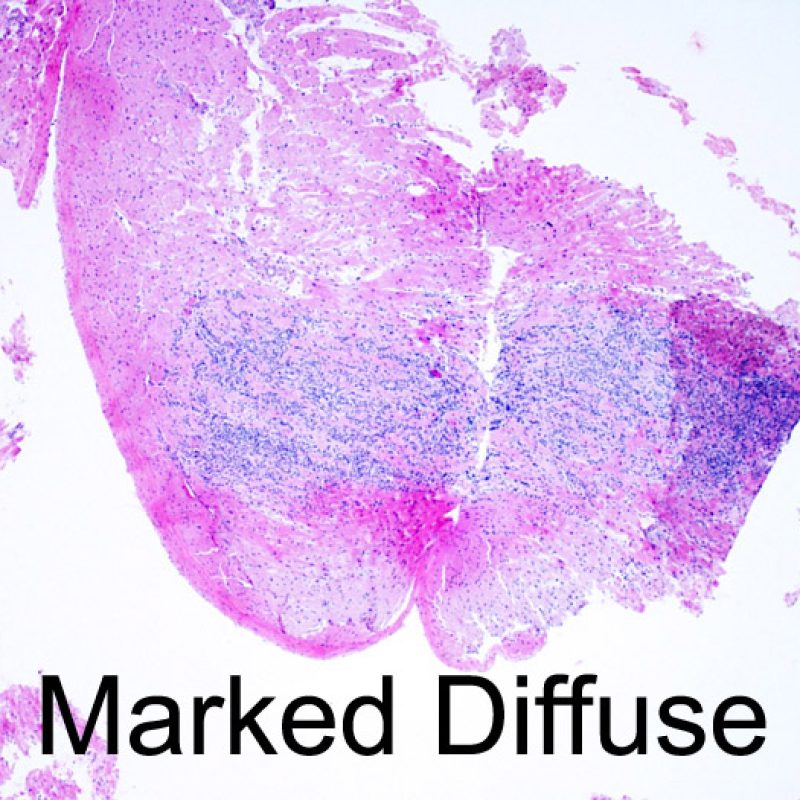
Severe rejection (3R) is a diffuse process of myocyte injury where it is difficult to make out distinct foci of injury. Numerous eosinophils and some neutrophils are typical in this level of rejection. Generally there is a very heavy inflammatory infiltrate across the biopsy tissues. Edema, hemorrhage and vasculitis can all be features of this injury. With modern treatment protocols, a 3R rejection is quite rare and is most likely to occur if a patient has stopped taking their anti-rejection medications. Generally patients who have a 3R rejection have concurrent myocardial dysfunction. It is always a good idea to convey to the clinicians the presence of 3R rejection on a biopsy as immediate treatment is warranted.
Antibody Mediated Rejection
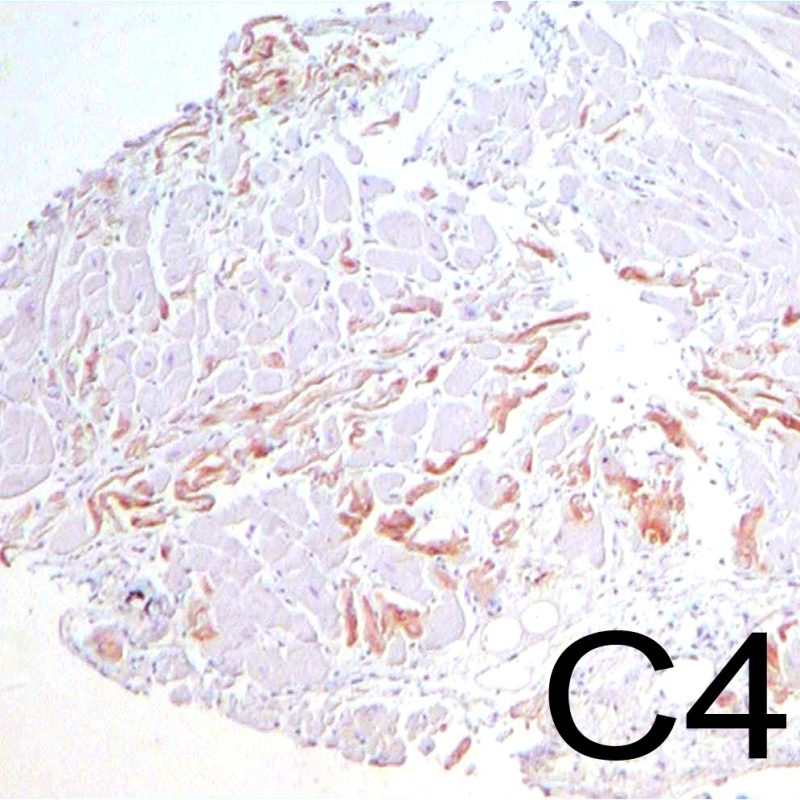
Antibody mediated rejection (AMR, AKA humoral rejection) is distinct from acute cellular rejection except in severe cases where the histologic findings may overlap. Histopathologic changes of AMR, seen on light microscopy, are endothelial cell swelling, macrophages filling small vessel lumens and edema. Staining for the complement split product C4d is generally used to identify the presence of AMR by immunohistochemistry or immunofluorescence.
Differences in ISHLT 1990 and 2004 criteria
As mentioned above, the ISHLT has had two separate grading criteria. The ISHLT-2004 criteria simplified the ISHLT-1990 criteria and eliminated categories that had no prognostic relevancy (ex ISHLT -1990 1A vs 1B and ISHLT 1990 grade 3B vs 4). The most controversial aspect of the change was the addition of the ISHLT-1990 grade 2 category (a single focus of myocyte injury) with the mild 1R category. Several institutions treat an ISHLT-1990 grade 2 differently than an ISHLT-2004 1R, by either instituting closer followup of the patient or treating the patient with increased immunosuppression. Thus it can be important to establish the significance of a single focus of injury on a biopsy at your institution to determine if this information needs to be conveyed to the clinicians when that finding is encountered.
Low Power
This section of the tutorial takes you through the observations made at low power (2x to 10x). It will cover topics such as observing cellularity, the interstium, myocytes and vasculature.
Biopsy Adequacy
The first low power biopsy assessment is to assure that adequate myocardium is present to evaluate for cellular rejection. As many heart transplant patients undergo multiple repeat biopsies, part or all of a biopsy piece may consist of a prior biopsy site. If organizing thrombus, granulation tissue, or areas of fibrosis with absent or disorganized myocytes are observed, a prior biopsy site is likely to be present. For adequacy, at least 3 biopsy pieces must be present, each with at least 50% of the surface area occupied by myocardium not altered by prior biopsy site. The presence of other non-myocardial tissues (valve leaflet, chorda tendinea, adipose tissue, etc.) should also be noted.
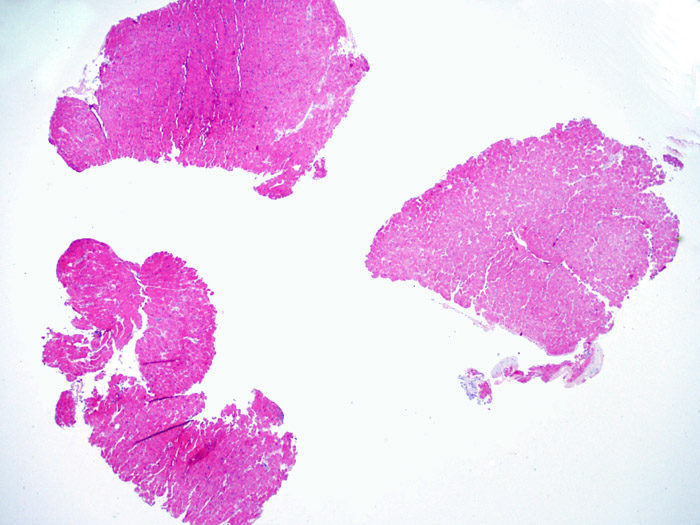
Overall biopsy cellularity
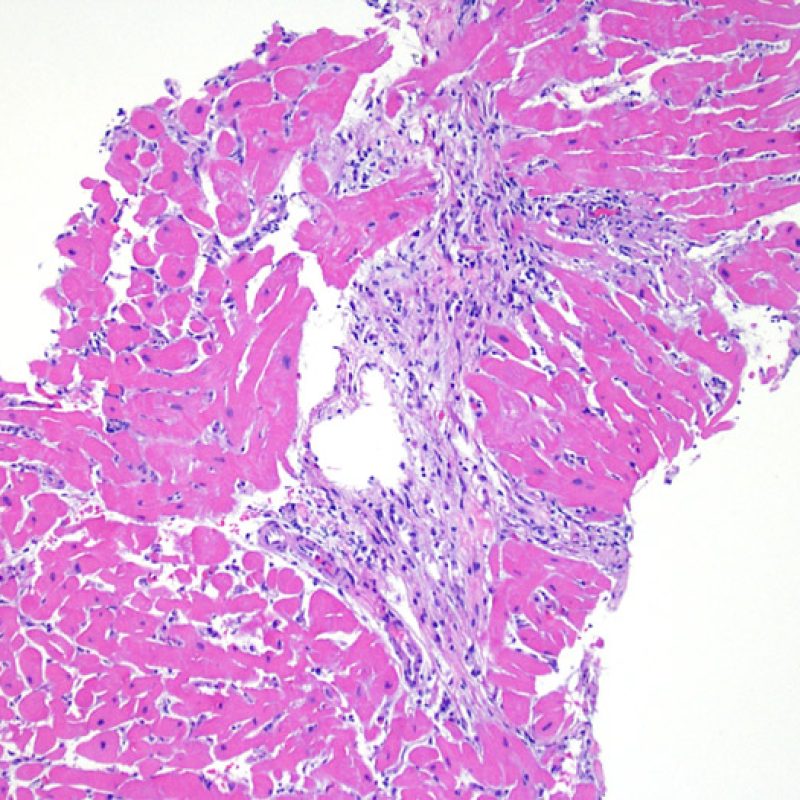
After determining biopsy adequacy, the major goals of low power examination are to: 1) note increased cells in the biopsy specimen; 2) determine the location and pattern of the cellular infiltrate; 3) get an overall feel for the amount of inflammatory infiltrate and 4) identify specific areas of increased cellularity to view at high power. The histological changes of acute cellular rejection are not uniform, and a low power scan allows the pathologist to focus on the worst lesional areas. Examination of tissue at several levels is critical for diagnostic accuracy.
Increased cellularity in EMB is readily identifiable in most cases. The distribution pattern of cellular infiltrates may be either focal or diffuse. At low power, it is generally possible to determine if the inflammation is perivascular or interdigitating among myocytes (perimyocytic) in a “chicken wire fence” pattern. Sometimes, this distinction, like many other morphological features, is subjective.
The location of infiltrates, and their relationship to myocytes, may suggest their underlying pathologies. The lymphocytic infiltrates in acute cell-mediated rejection (ACR) are found around cardiomyocytes (perimyocytic) or around small arterioles (perivascular). If the infiltrate is located primarily within the endocardium or in fibrous tissue, alternate etiologies such as ischemic injury, Quilty infiltrates or previous biopsy site should be considered. Endocardial lymphocytic infiltrates may also run along the surface, or near the endocardial surface in a linear fashion. These can be difficult to distinguish from a Quilty effect and tend to be be seen in association with perimyocyte infiltrates in higher grades of ACR.
Large, nodular, collections of lymphocytes along the endocardium, are typical of “Quilty” effects. For Quilty lesions in the myocardium or extending into the myocardium from the endocardium, low power observation of dense lymphocytic infiltrates with pushing borders and a relatively sharp interface with adjacent myocytes should suggest the correct interpretation. All perimyocytic lymphocytic infiltrates should be evaluated at higher magnification for evidence of myocyte injury.
Myocytes
The identification of “myocyte injury” is sometimes a challenging task during EMB examination. The “injury” covers a wide spectrum of changes with cell death at one end and subtle degenerative change at the other. Most of these changes need to be visualized at high power for accurate diagnosis.
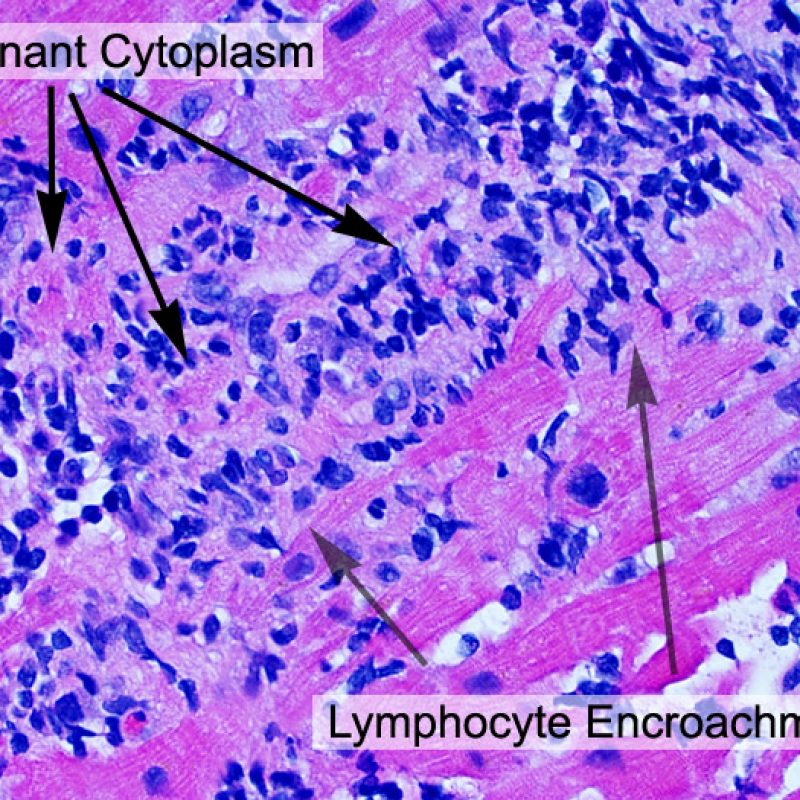
Circumstantial evidence of myocyte injury, seen at low power includes encroachment of inflammatory cells onto myocytes or frankly overlying myocytes. The infiltrating inflammatory cells cause irregularity of myocyte borders and distortion of the normal myocardial architecture. In practice, inflammatory cells inside or close to myocytes are very convenient biomarkers that call for more careful examination. Caution should be used in any attempt to evaluate lymphocytes in relation to myocytes in areas of crush artifact from the bioptome.
Occasionally, ischemic injury (peri-operative or late ischemia due to allograft vasculopathy) induces foci of cardiomyocyte coagulation necrosis. Small foci involving few myocytes may not be distinguishable from ACR at low power. Larger foci will be readily evidenced by coagulative necrosis, typically with myocytes devoid of nuclei and variable degrees of myocyte resorption. Regardless of size, ischemic foci are often accompanied by a modicum of surrounding macrophages, lymphocytes and rarely neutrophils. In some cases these areas may be sharply demarcated and stellate in shape. The necrotic myocytes can be highlighted by trichrome staining which will stain these myocytes purplish.
Vacuolated myocytes may reflect myocytolysis secondary to chronic ischemia. However, contraction band artifact, particularly when seen in myocytes cut in cross-section may mimic myocytolysis. Careful low power assessment for the “checkerboard” pattern of myocytes randomly sectioned through condensed contractile elements, alternating with hyperlucent, lytic appearing areas should raise red flags and suggest a diagnosis of chronic ischemia.
Interstitium
Other than increased interstitial cellularity (discussed above), low power changes that can be observed in the myocardial interstitium are mainly edema and fibrosis. Edema is seen in the setting of severe ACR (Grade 3R), heart failure and/or leaky, injured vasculature with antibody mediated rejection. The interstitium has a pale basophilic, slightly bubbly appearance, evident in perivascular and/or perimyocyte spaces. The findings should be correlated with high power evaluation of the microvasculature for features of antibody mediated rejection. Edema should not be confused with artifactual, biopsy or histological preparation-induced spaces. These can be be small or large empty areas between myocytes without any of the wispy extracellular matrix seen in edema.
Increased interstitial fibrous tissue is most often in the form of broad areas of loss of myocytes with replacement fibrosis. These lesions are usually related to biopsies obtained within, or directly adjacent to, prior biopsy sites. Specific features of biopsy site changes are discussed elsewhere. Scarring related to chronic and/or healed myocardial ischemia may be difficult to distinguish from prior biopsy sites. Low power observation of “stellate shaped” scars should prompt high power evaluation for additional features of chronic ischemia. Regardless of the presence of these findings, interstitial fibrosis is not a feature of ACR.
Presence of vasculature
Evidence of cardiac allograft vasculopathy can rarely be noted on EMB and can easily be confused with the artifactual telescoping of blood vessels. Large vessels, particularly when associated with adipose or neural tissue should prompt high power evaluation for evidence of perforation. Pathologic changes in the vasculature are best evaluated at high power.
High Power
After a slide is visually scanned at low power (4x, 10x objectives) a pathologist typically moves to high power on areas that contain an inflammatory infiltrate. Generally this is with a 20x objective. At this power, one is investigating primarily for myocyte injury in areas of lymphocytic infiltrate. There is no strong indication to use the high power objectives on areas of normal appearing myocytes observed at low power. This section of the tutorial takes you through the observations made at high power. It will cover the topics of myocyte injury, identification of the infiltrating cells, and interstitial findings.
Confirmation of myocyte injury
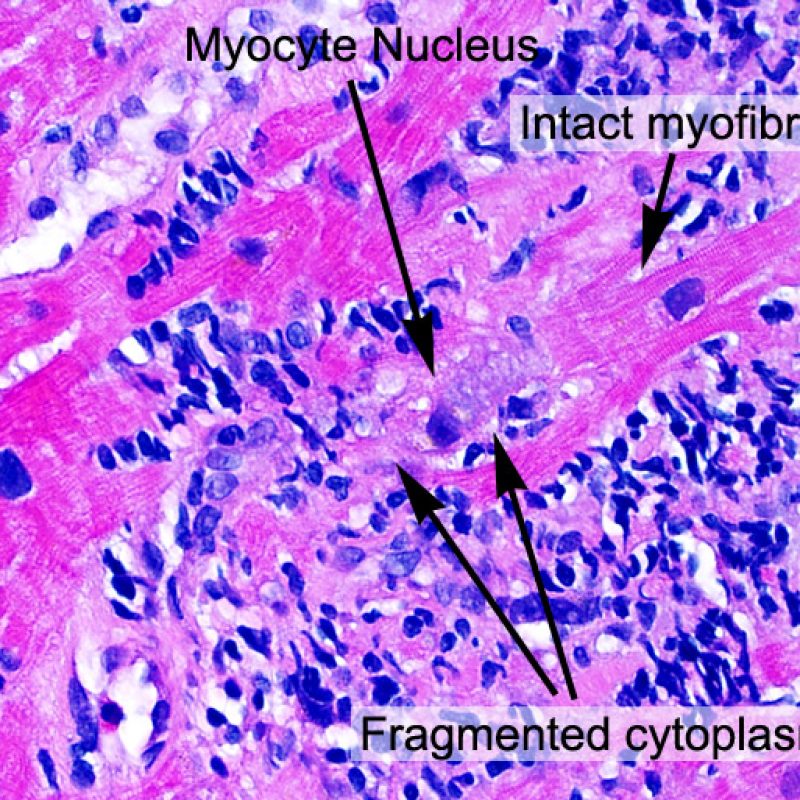
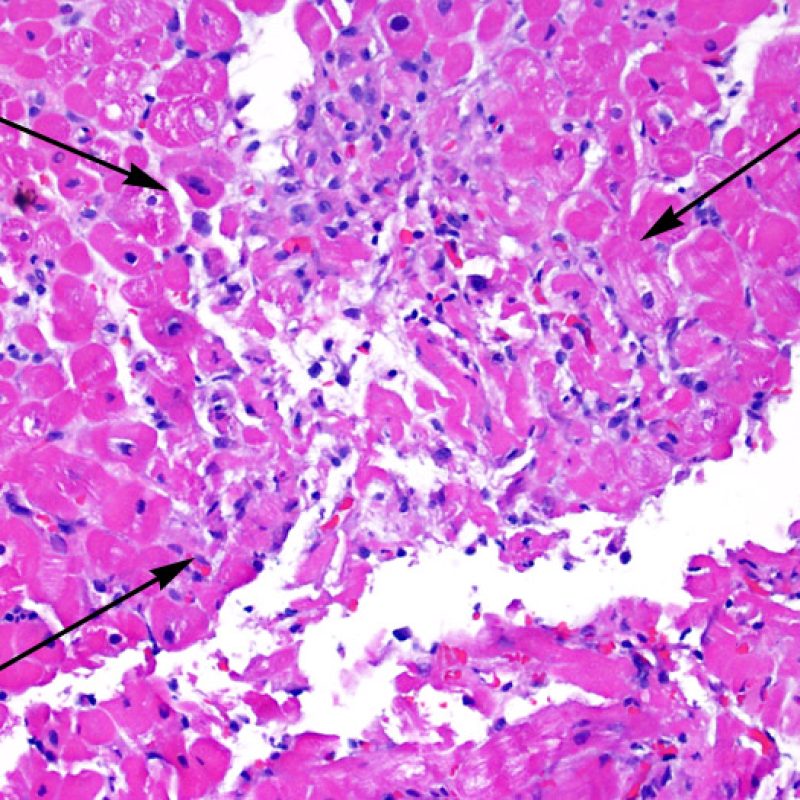
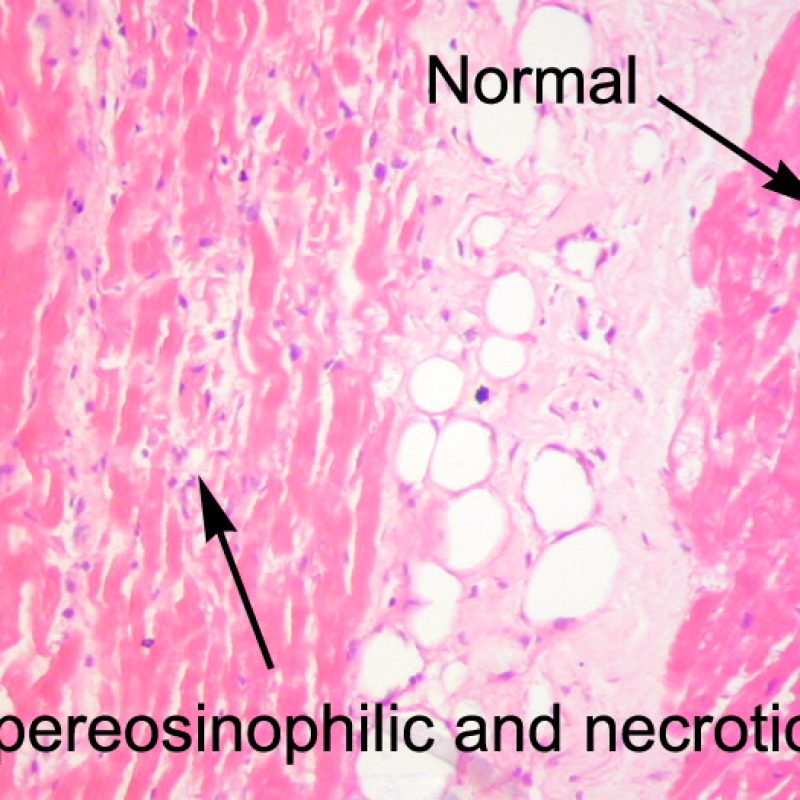
Once the presence of a lymphocytic infiltrate is established, the next step would be to look for myocyte injury. Myocyte injury (ie damage/necrosis) that is associated with acute rejection may be difficult to recognize, and other changes can be mistaken for myocyte injury (poor fixation). Myocyte injury is often subtle and best appreciated by observing the same area across multiple levels. Some morphological features of myocyte injury include: vacuolization, perinuclear halo, myocyte nucleus without surrounding cytoplasm, lymphocyte encroachment onto the myocyte with irregular myocyte borders, and myocyte dropout (areas of heavy inflammation and matrix material where a myocyte should have been). Hypereosinophilia and nuclear pyknosis are also good indicators of myocyte injury. In the revised working formulation, milder types of acceptable myocyte injury include myocytolysis and coagulative necrosis.
Another clue for myocyte injury is a change in the sarcomere structure. If one can see normal myofibril patterning in the myocyte, that myocyte is not injured. If that patterning is gone and the cytoplasm becomes hypereosinophilic and clumpy, there is a good chance that myocyte is injured. Masson trichrome stain can highlight early myocyte injury, in which case injured myocytes are shrunken and have more of a blue-gray color instead of the normal eosinophilia seen in normal myocardium.
All of these changes must be seen in the presence of adjacent lymphocytes. If lymphocytes are not present, then the myocyte injury is not caused by cellular rejection and would be the result of some other process such as ischemic injury that can be seen in the early weeks following transplantation.
It is always a good idea to look at multiple levels of the potentially injured area as confirmation of the myocyte injury as sometimes a single histologic section can be misinterpreted as injury.
Identification of interstitial cells
If one looks beyond the myocytes, one can see a variety of additional cell types in the biopsy material. The most important would be the cellular infiltrate of acute cardiac rejection, which is principally T cells (CD4+ and CD8+), with fewer macrophages and occasional eosinophils. A substantial increase in activated B cells and natural killer cells has been seen in moderate to severe rejection.
Not all nuclei seen within the interstitium represent lymphocytes, however, and high magnification may be needed to identify the exact nature of the “infiltrate” to exclude cellular rejection. Other cellular components of the myocardial interstitium include fibroblasts and myofibroblasts, primitive mesenchymal cells, dendritic cells, mast cells, endothelial cells and pericytes. Scattered lymphocytes and occasional macrophages can also be seen in normal myocardium. Generally, only lymphocytes cluster in the myocardium.
Nature of the inflammatory infiltrate
The type of inflammatory cells seen in endomyocardial biopsies from cardiac transplant recipients is important in distinguishing an infiltrate as being due to rejection versus another process, as well as helping to determine the grade of rejection. Acute cellular rejection is always characterized by the presence of lymphocytes (T cells). Activated lymphocytes, which are more prominent in higher levels of rejection, have increased cell cytoplasm.
In the appropriate setting, a mixed inflammatory cell infiltrate with neutrophils and eosinophils may be indicative of higher grades of cellular rejection (3R). Neutrophils can also be seen in heart biopsies that have ischemic necrosis which can be present for several weeks after transplantation.
Eosinophils can be found in moderate (2R) or severe (3R) grades of rejection in which there is also myocyte necrosis. Generally they are rare and scattered. Eosinophils are almost never a feature of mild (1R) rejection and their presence should serve to increase one’s awareness that moderate or severe (2R & 3R) rejection, maybe present.
Eosinophils can also be seen in hypersensitivity myocarditis most often related to pharmacologic agents. In this scenario, the eosinophils make up a large ratio of the infiltrating cell mix and often cluster. The inflammatory infiltrate in hypersensitivity myocarditis also includes lymphocytes, plasma cells, and macrophages. Although focal myocytolysis can be seen in hypersensitivity myocarditis, myocyte necrosis is not a prominent feature. Eosinophils in EMB can also be seen in parasitic infection.
Nature of the inflammatory infiltrate
The interstitium should be evaluated at high power for the presence of cellular material and edema. The presence of a debris field, nuclear debris or other amorphous (“dirty”) basophilic and eosinophilic material, in areas of heavy infiltrate is consistent with myocyte injury and cellular apoptosis. Early after transplantation, it is more often associated with peri-transplant ischemic injury. However, in late biopsies it is a useful histologic finding in areas concerning for rejection.
Interstitial edema in a cardiac transplant biopsy may be a sign of antibody-mediated rejection. However, separation of myocytes can be seen as an artifact of handling and processing of the biopsy and therefore simulate edema. With true edema, myocytes are separated by whispy eosinophilic strands of matrix. Additionally, in the setting of antibody-mediated rejection, other findings should also be present, such as prominent capillary endothelial cells, interstitial macrophages, and/or hemorrhage.
Nature of the inflammatory infiltrate
Focal or diffuse subendocardial vacuolization of myocytes, or myocytolysis, may be a marker for reversible ischemic injury. Myocytolysis is a clearing of the sarcoplasm and nuclei, with nuclear enlargement and prominent nucleoli. If seen in endomyocardial biopsy samples from cardiac transplant recipients who are more than a year from transplantation, this finding could be a marker for accelerated coronary artery narrowing that occurs in transplanted hearts.
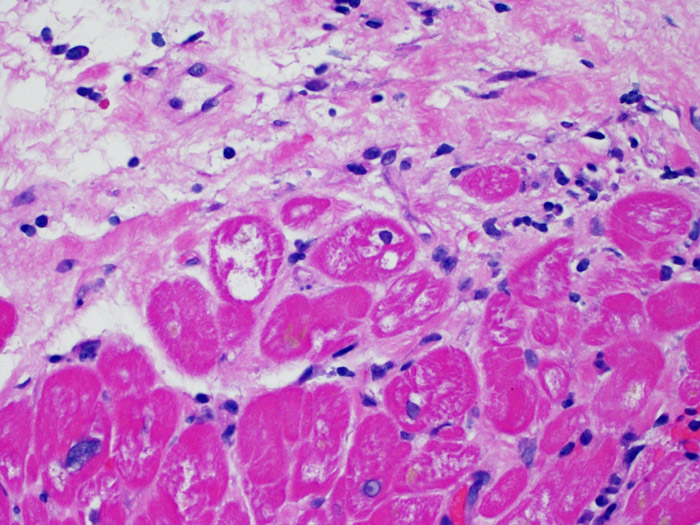
Endothelial cell changes (in AMR)
Criteria for making the diagnosis of antibody-mediated acute rejection are presented elsewhere in this tutorial. Among the findings on H & E-stained slides that may suggest AMR, the low power prominence of capillaries and venules may be helpful. High magnification is useful for examination of endothelial cells, which in AMR, are large and prominent (“swollen”), with enlarged and hyperchromatic nuclei. These findings support the presence of AMR and warrant further investigation to asses for the presence of immunoglobulin and complement.
Rejection
This section of the tutorial covers the main findings used to make the diagnosis of acute cellular rejection. It also has a brief word on antibody mediated rejection, which will be covered in greater detail in a forthcoming tutorial. It will consolidate the key concepts of the low power and high power sections.
Lymphocytic infiltrate
A lymphocytic infiltrate is the key histologic finding that indicates the process being observed is acute cellular rejection. At low power, this is seen as an increased area of cellularity. At high power lymphocytes must be evaluted for their injury to myocytes. Activated lymphocytes, which are associated with injury can have enlarged nuclei and increased cytoplasm compared to quiescent lymphocytes.
Myocyte injury
Myocyte injury is the key feature to discriminate between low and high grades of rejection. Higher grades of rejection are 2R and 3R. Myocyte injury is best appreciated at high power where a variety of features can be noted. These include hypereosinophilia, nuclear pyknosis, myocytolysis, coagulative necrosis, vacuolization, perinuclear halo, and lymphocyte encroachment onto the myocyte. Sometimes myocyte injury is appreciated as an area with heavy lymphocytic inflammation where there has been myocyte dropout (empty spaces where myocytes should be) or “bits of myocytes” from the destructive process.
Antibody Mediated Rejection
Antibody mediated rejection (AMR, AKA humoral rejection) is distinct from acute cellular rejection except in severe cases where the histologic findings may overlap. Histopathologic changes of AMR, seen on light microscopy, are endothelial cell swelling, macrophages filling small vessel lumens and edema. Staining for the complement split product C4d is generally used to identify the presence of AMR by immunohistochemistry or immunofluorescence.
Non-Rejection Findings
This section of the tutorial will identify a number of non-rejection findings that can be seen in these biopsy samples. Some of these findings are common, while some are exceedingly rare. Many of these findings can be easily confused with rejection and it is critical to not overcall rejection based on these histologic patterns.
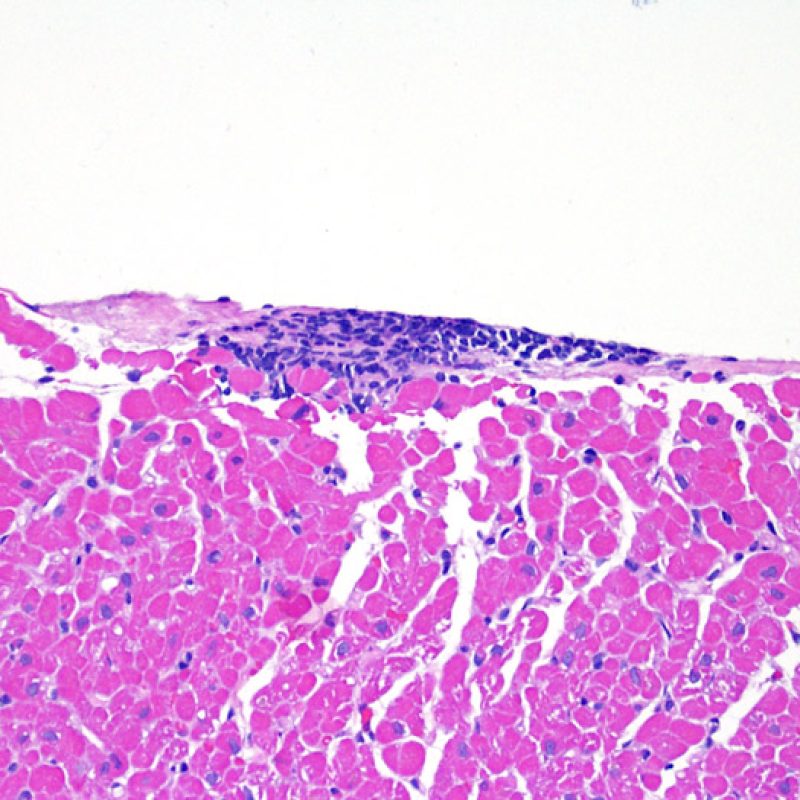
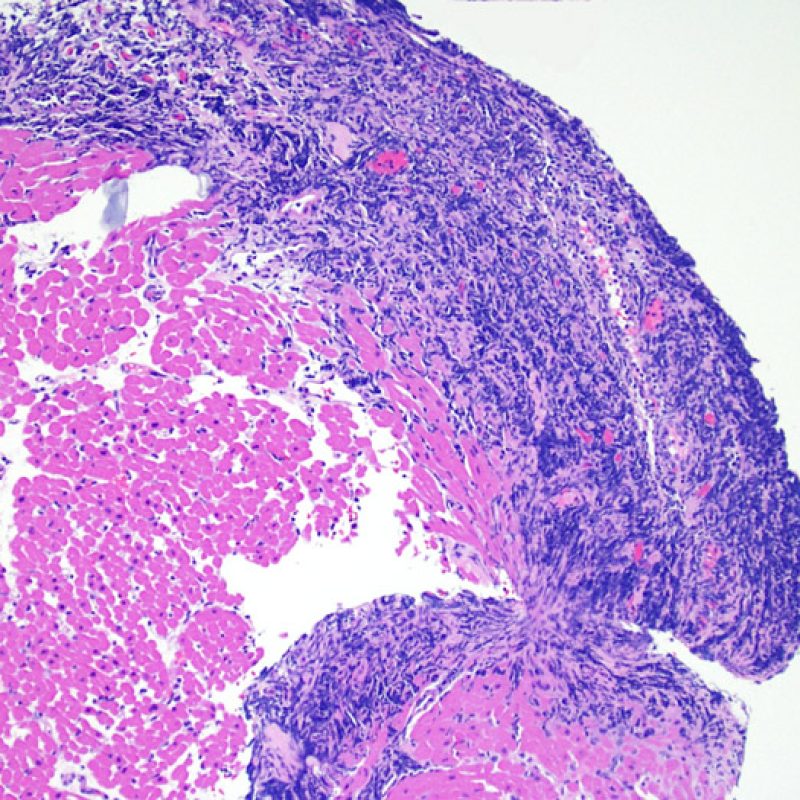
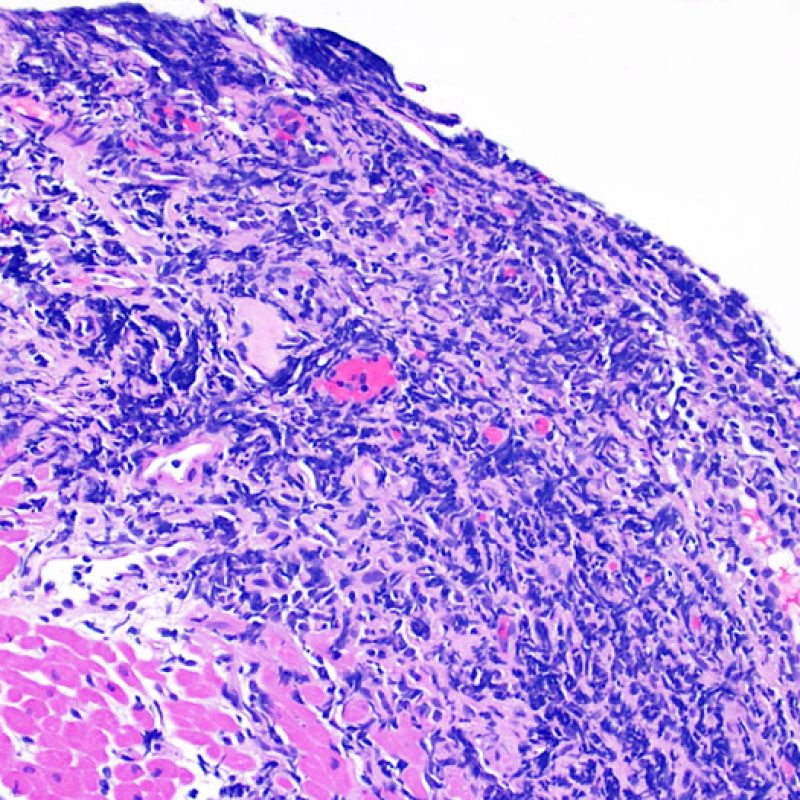
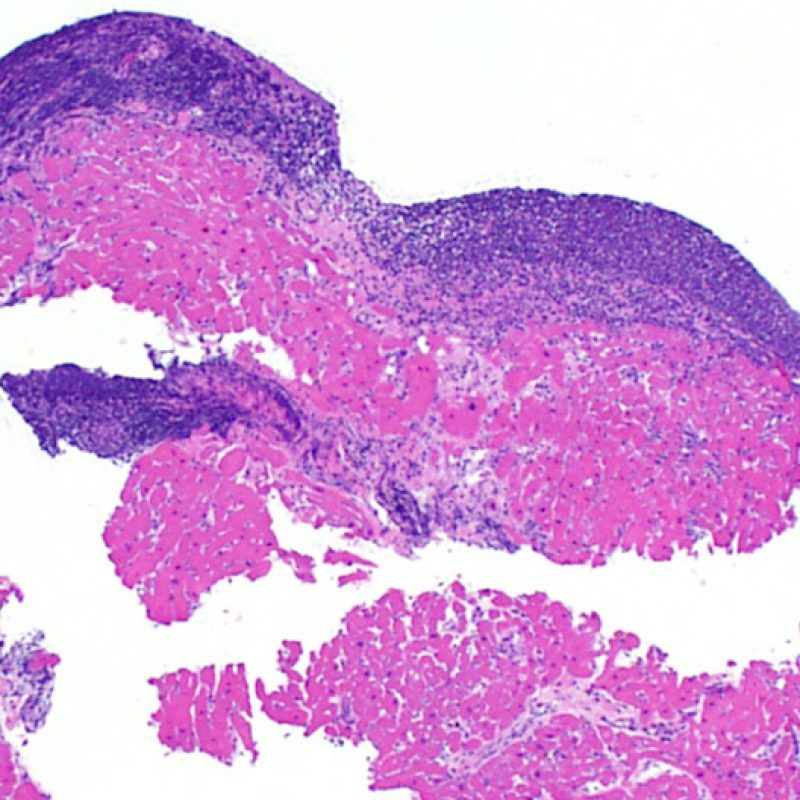
Quilty Effect
Endomyocardial biopsies in ~50% of patients with cardiac allografts may have focal, dense endocardial collections of lymphocytes that can extend into adjacent myocardium. These common changes are termed Quilty effect (formerly Quilty lesion, named after the first patient in whom they were described). These lesions consist of a mix of B lymphocytes and T lymphocytes and occasionally dendritic cells. Small vascular channels (capillaries) can be noted within some of the inflammatory infiltrates.
The pathogenesis of the Quilty effect is not known, but hypotheses have included cyclosporine-based immunosuppressive regimens, idiosyncratic responses to cyclosporine A, and concomitant Epstein-Barr virus infection. The Quilty effect is not seen in hearts from patients who received cyclosporine A for other solid organ transplants (i.e. kidney, liver). Many groups have investigated the relationship between Quilty effect and grade of cellular rejection, viral infection, AMR, and/or development of graft vasculopathy. Studies both supporting and refuting associations with these other findings make this a continuing area of discovery. Overall, there is not a consensus of an association between Quilty effect and any key histopathologic or outcome data.
Quilty effect can be confined to the endocardium, or it can extend into adjacent myocardium. Some large Quilty effects do interdigitate amongst underlying myocytes resulting in peri-Quilty myocyte injury. This mimicker of rejection is believed to be a frequent etiology of the the ISHLT-1990 grade 2 lesions. In this scenario, a distinction from acute cellular rejection must be made. This may require multiple sections through the tissue block to establish that it has an endocardial component (although acute cellular rejection can also have an endocardial distribution). When present, small vessels or capillaries within the inflammatory infiltrate can be very helpful in recognizing Quilty effect. Additionally, the stroma among myocytes in Quilty effect may appear more fibrous.
In some cases, lymphocyte typing by immunoperoxidase techniques may be required, in which case Quilty will have a mixture of B and T lymphocytes and macrophages, unlike acute cellular rejection in which T cells predominate. A CD21 stain can also be used. When positive, it is a strong marker of Quilty effect. A negative CD21 is not prognostic.
If Quilty effect is seen within other pieces in the biopsy, it may be more likely that a focus of inflammation within the myocardium is also Quilty, although acute cellular rejection cannot be totally excluded on that basis alone. As a Quilty effect heals, small scars simulating previous biopsy sites can be seen. The scars with Quilty tend to have more even, linear borders in contrast to scars from previous biopsies that have a more uneven interface.
Ischemic (Peri-transplant) injury
Ischemic injury (peri-transplant injury) is usually noted in the biopsies taken during the first 6 weeks after transplant but can be present for up to 3 months. This process can be difficult to distinguish from acute cellular rejection. Due to the ischemia of the heart as it is moved from the donor to the recipient, some variable number of myocytes will die from this process. Myocyte death is generally found in clusters (large or small). In the inital biopsy, myocyte death may be very subtle with only increased myocyte cytoplasmic eosinophilia and anucleation being apparent. Dead myocytes will stain strongly for C4d by immunohistochemistry or immunofluorescence. This should not be confused with antibody mediated rejection. Late myocyte death can be seen, but can be distinguised from acute cellular rejection by a lack of lymphocytic infiltrate. Due to the strong immunosuppression, inflammation may not be observed early in the process.
Generally 2-4 weeks after transplant, the maximal inflammation process is present. This is primarily a macrophage-mediated response. This inflammation can be extensive and associated with myocyte injury. The key feature to distinguish this from acute cellular rejection is a mixed inflammatory infiltrate versus a lymphocyte-rich infiltrate. Ischemic injury can also have a “dirty” interstitial background. When there is both acute cellular rejection and ischemic injury in the same biopsy, making a distinction can be difficult. It is very easy to overcall rejection at this time point due to the dual processes, but since most patients will return for a repeat biopsy within a week or two, caution is advised.
After the first month, most ischemic injury looks like a typical resolving small infarction with pigment-laden macrophages, loose connective tissue and a regional loss of myocytes. This is not as easily confused with acute cellular rejection but it is difficult to distinguish from a previous biopsy site.
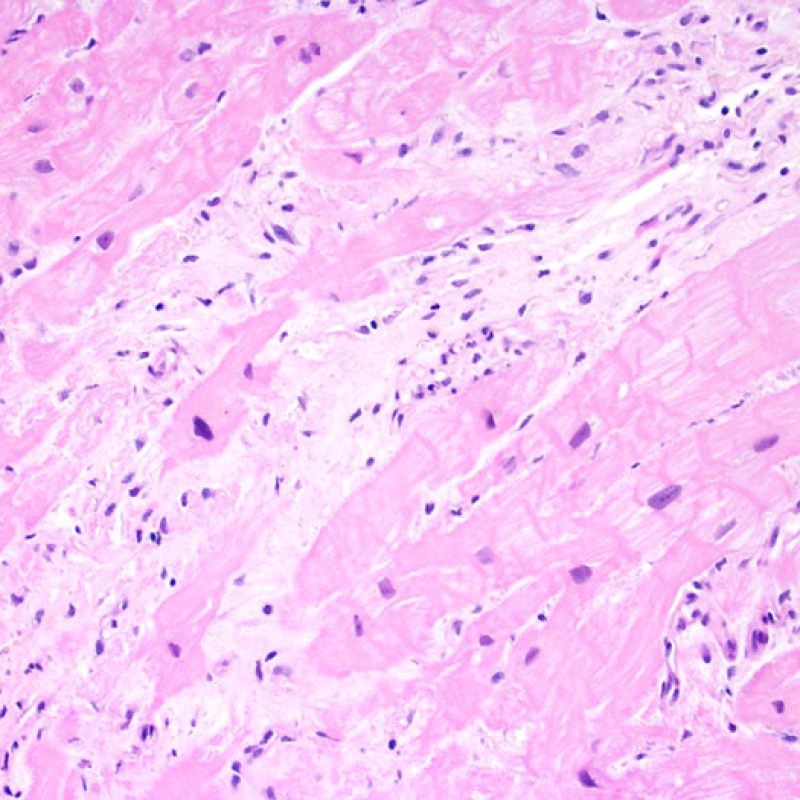
Biopsy site changes & thrombus
Due to the architecture of the right ventricle, it is not uncommon for the bioptome to be guided to the same or similar locations and injury from previous biopsies in cardiac transplant patients should be recognized. The appearance of a previous biopsy site will vary depending upon the interval between the current and previous biopsies. A recent biopsy may result in a focal endomyocardial defect with an overlying fibrin-platelet or organizing thrombus and some inflammatory cells (macrophages, T, and B cells). During the reparative stage, it may consist of granulation tissue, and eventually a focal endocardial scar will be seen. The myocytes adjacent to a scar will have focal disorganization and/or irregular hypertrophy. They may appear as myocyte disarray. In situations in which patients undergo repeated endomyocardial biopsies, such as cardiac transplantation, the chance of obtaining a previous biopsy has been reported to range from one in seven up to nearly 70% of biopsies.
Inflammation in areas of biopsy site changes is not acute cellular rejection and should not be graded as such. Thus a biopsy may have no rejection (0R) but have an area of inflammation associated with a biopsy site.
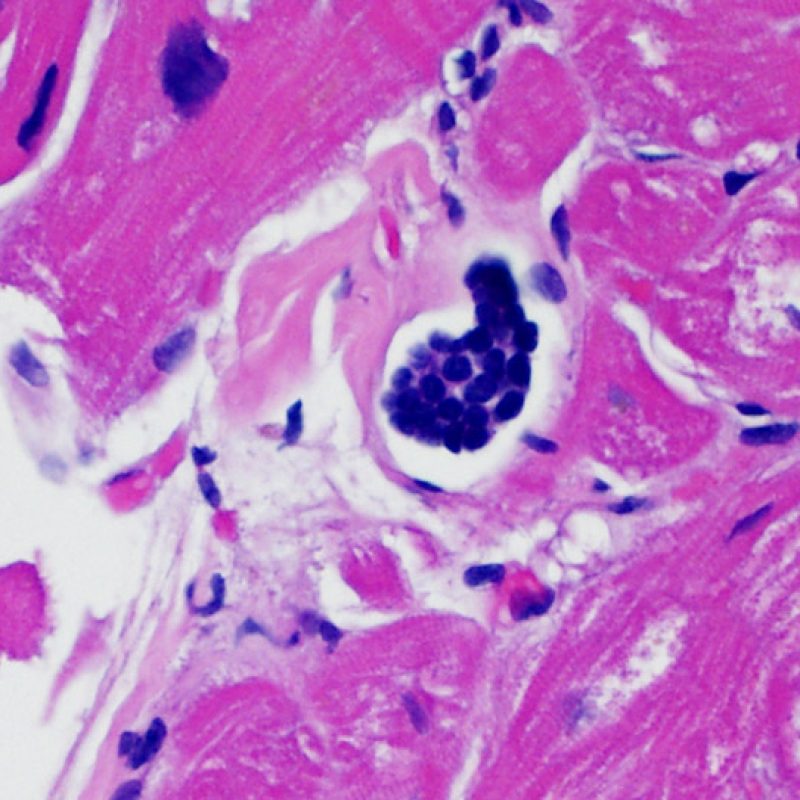
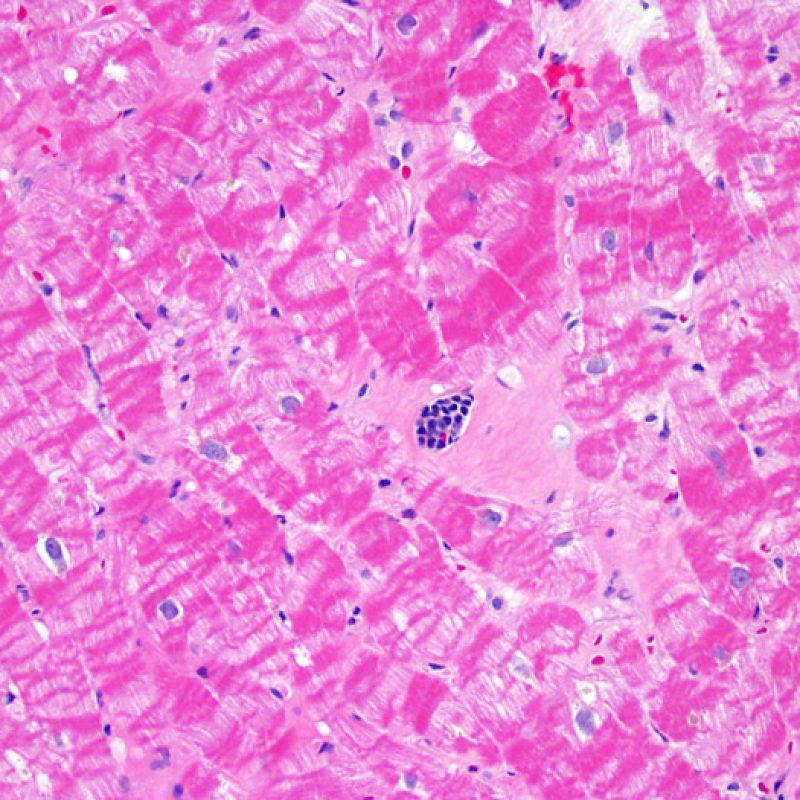
Intravascular lymphocytes
In some individuals, collections of lymphocytes filling a lymphatic channel appear. The etiology of this finding is unknown. This finding is not related to acute cellular rejection and does not need to be reported.
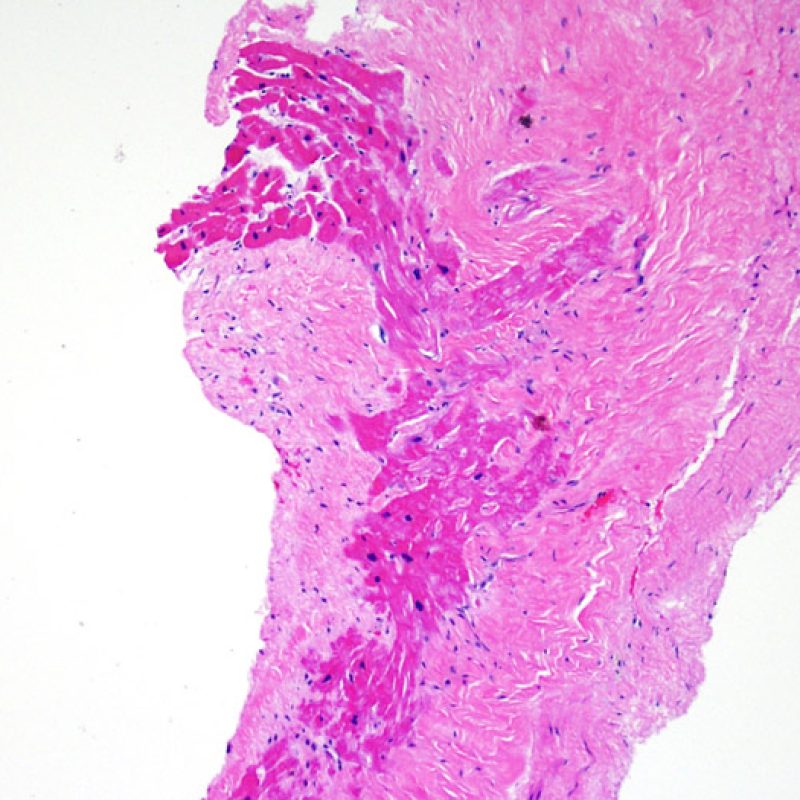
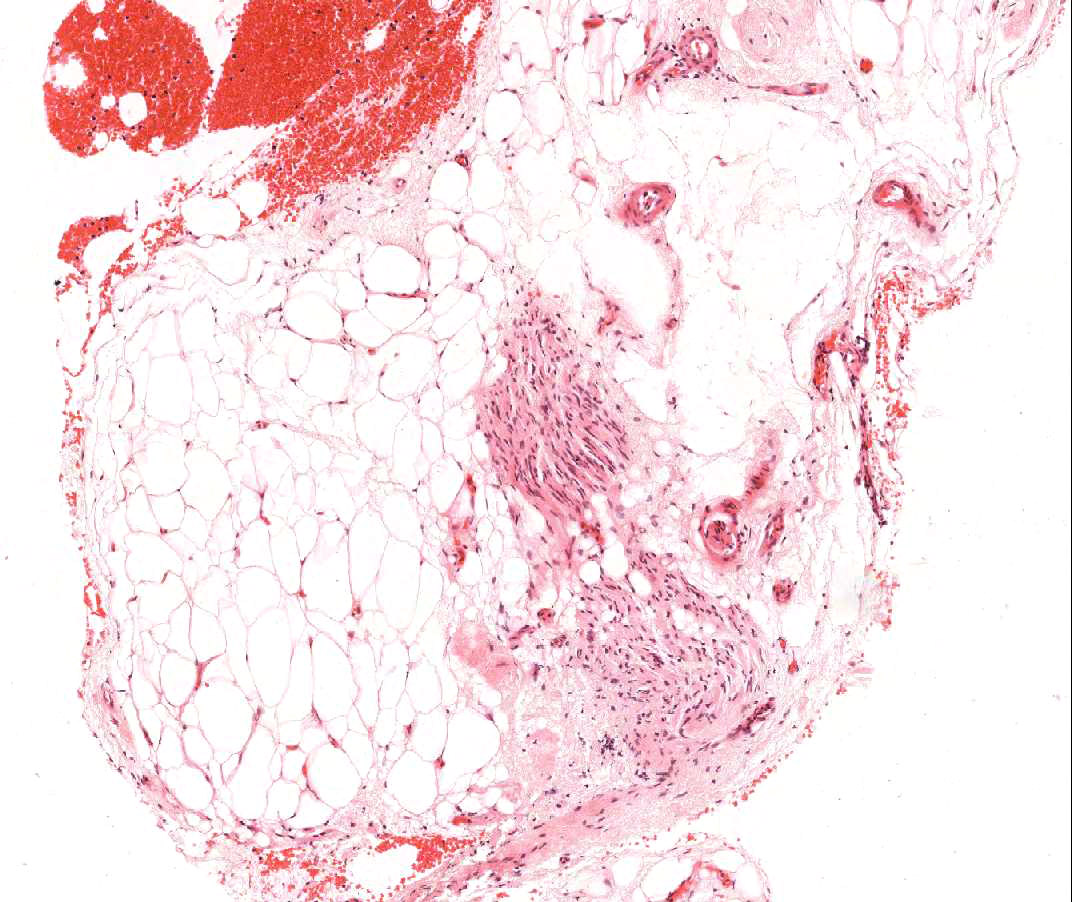
Adipose tissue and mesothelial cells
Adipose tissue is a normal, minor component of the myocardium, often appearing adjacent to larger arterioles. Occasionally, larger collections of adipocytes, or frank adipose tissue is seen in biopsy samples. Frequently, they will have small portions of non-compact myocardium attached. These samples come from free wall of right ventricle and, apart from not being suitable for assessment of acute rejection by virtue of their site, they have little significance. Occasionally the presence of adipose is more ominous. When there are strips of mesothelium either attached to the fat or found separately in the block, or when small nerves are noted within the fat, this suggests the source of the tissue is the epicardial fat. This indicates perforation of the right ventricle and the clinician should be contacted urgently to be informed of this fact because of the risk of significant hemopericardium. In most heart transplant patients the pericardium has adhered to the heart, making the risk of fatal hemopericardium significantly reduced relative to a similar finding in a native heart biopsy.
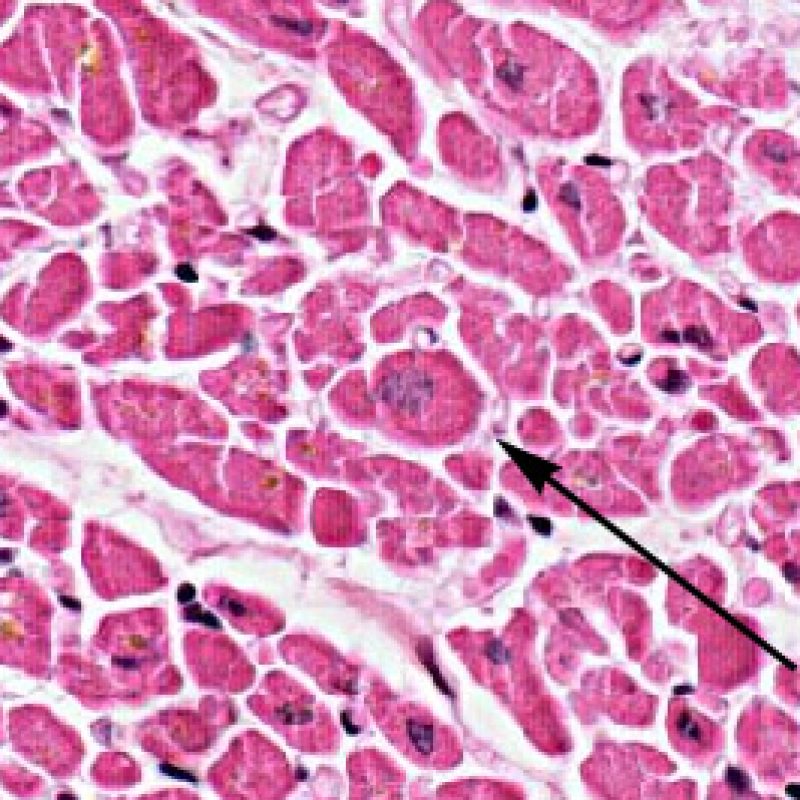
Myocyte calcification
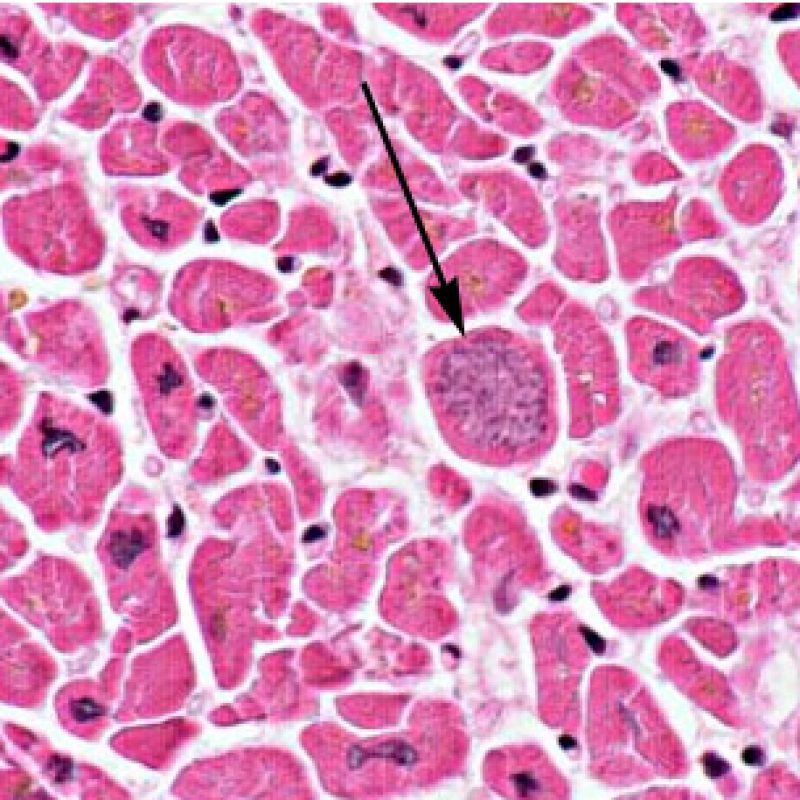
Myocyte calcification is an extremely rare finding at heart transplant. The etiology is believed to be transient hypercalcemia during the transplantation event resulting in metastatic calcification of myocytes. It can also be observed in old biopsy sites. Calcification is more likely to occur in the setting of acute or chronic renal failure. Histologically this appears as calcification of myocytes either individually or in clusters. Increased fibrosis may occur in these sites, but inflammation is not typical. Although extensive cardiac calcification may negatively affect cardiac function, it is not associated with acute cellular rejection.
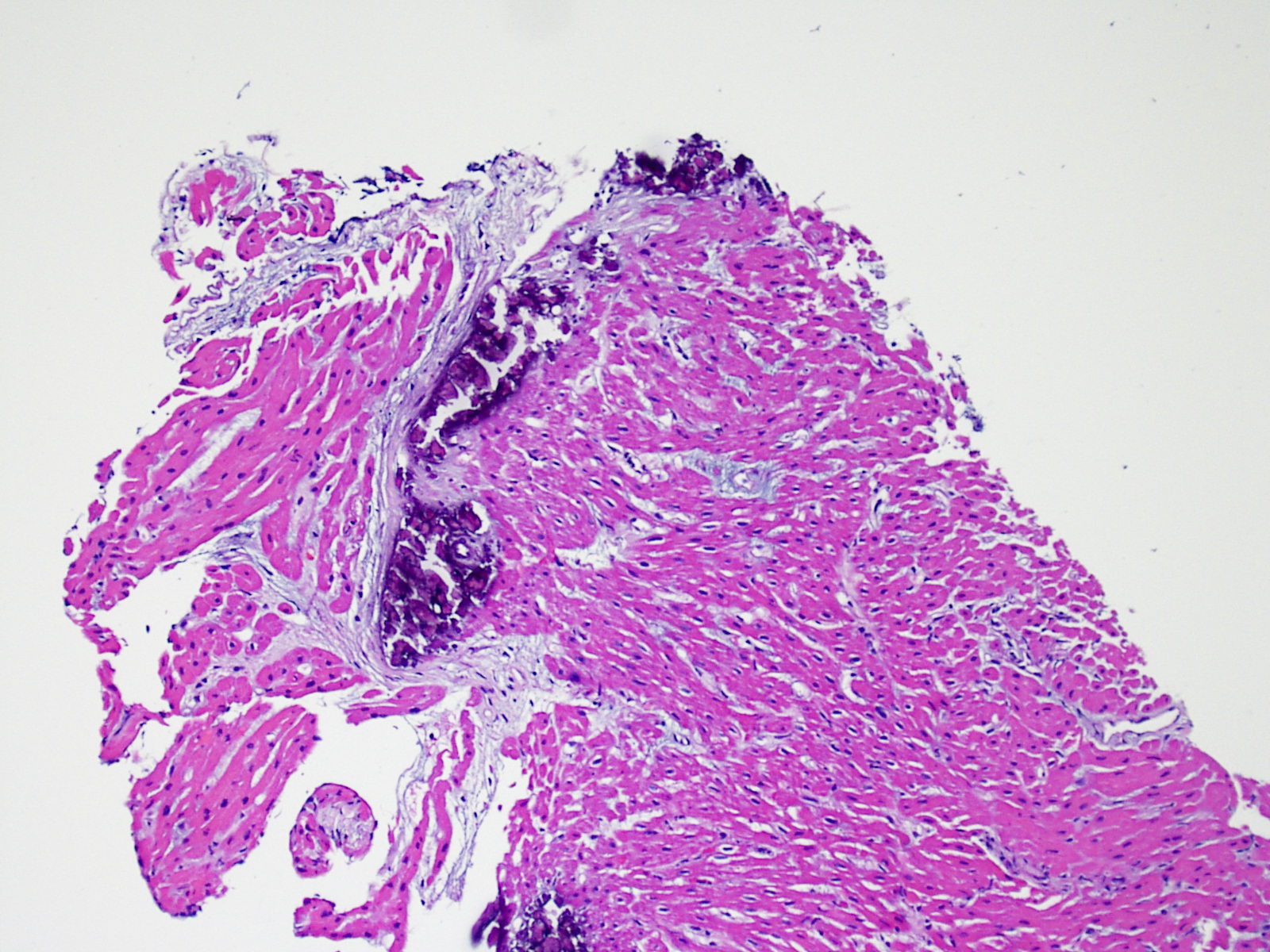
Disease recurrence
Some disease processes that result in heart failure have been reported to recur in the transplanted heart. These include sarcoidosis, giant cell myocarditis, amyloidosis, Chagas and light chain deposition disease. A pathologist should be aware of any of these reasons why a patient was transplanted and be aware of a return in these diseases. Sarcoidosis and giant cell myocarditis may both particularly mimick acute cellular rejection.
Infection
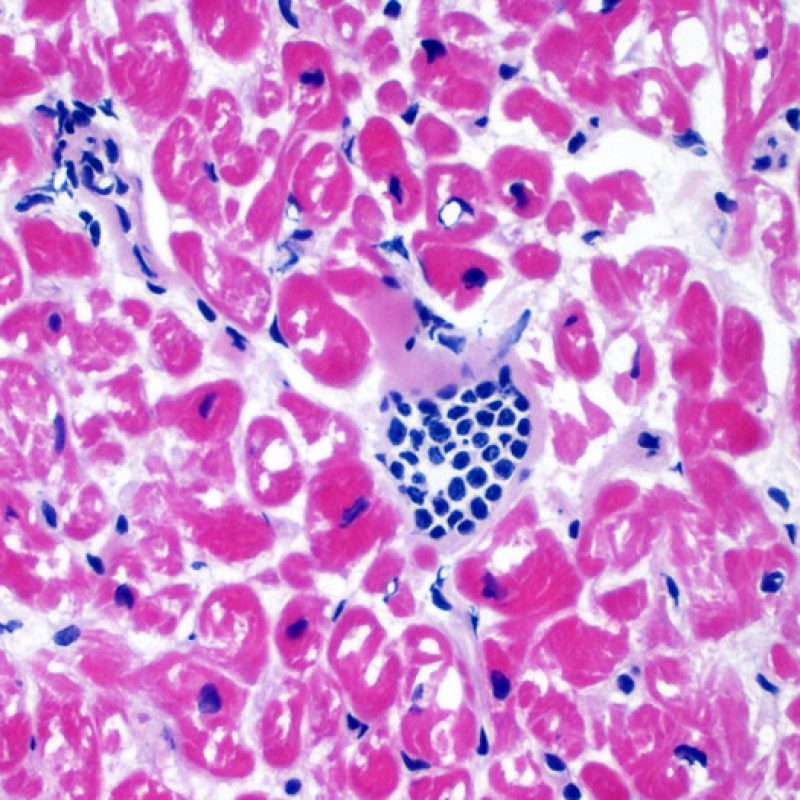
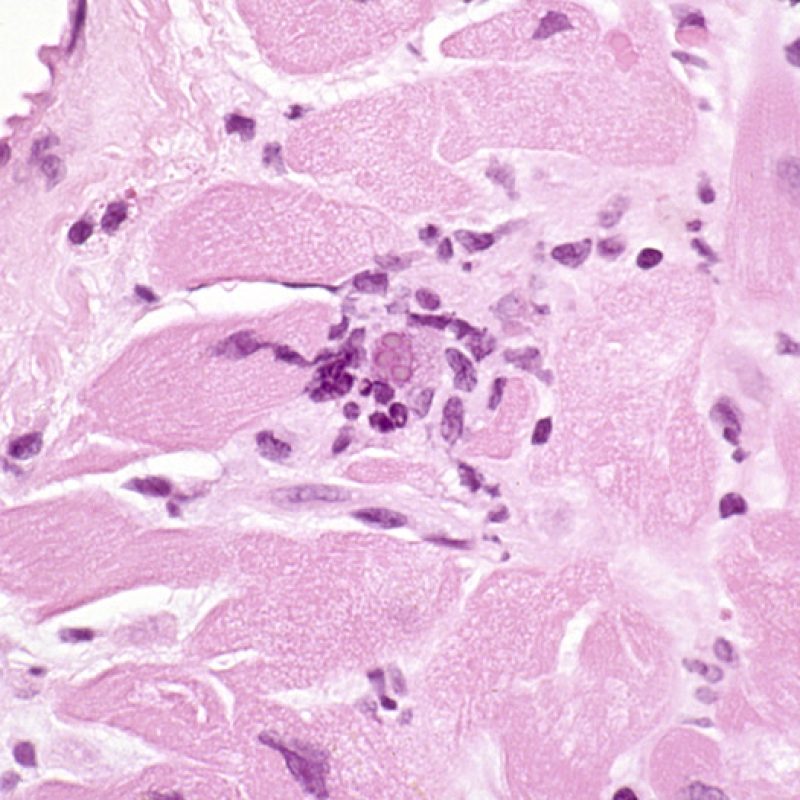
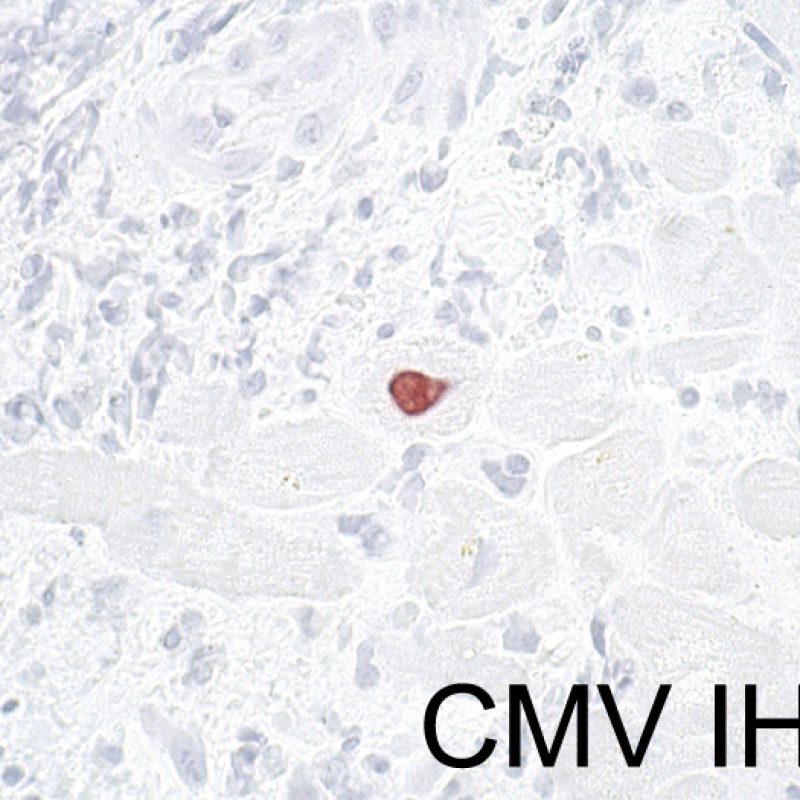
It is very unusual to identify an infection in an endomyocardial biopsy, despite transplant recipients being at elevated risk of infection due to immunosuppresion. Cytomegalovirus (CMV) has been reported in heart biopsies, however CMV status is usually better identified by serology (only if the recipient was initially CMV negative prior to transplantation) or in other tissues (ex. esophagus). However, the presence of viral inclusions may be identified on the biopsy material. A CMV or other viral immunohistochemical stain can then be obtained. If infection is present, that information should be conveyed to the clinician but should not be confused with acute cellular rejection. Toxoplasmosis may also be seen in the transplanted heart. Bradyzoites can fill myocytes without a significant inflammatory reaction. This can also be detected by immunohistochemistry.
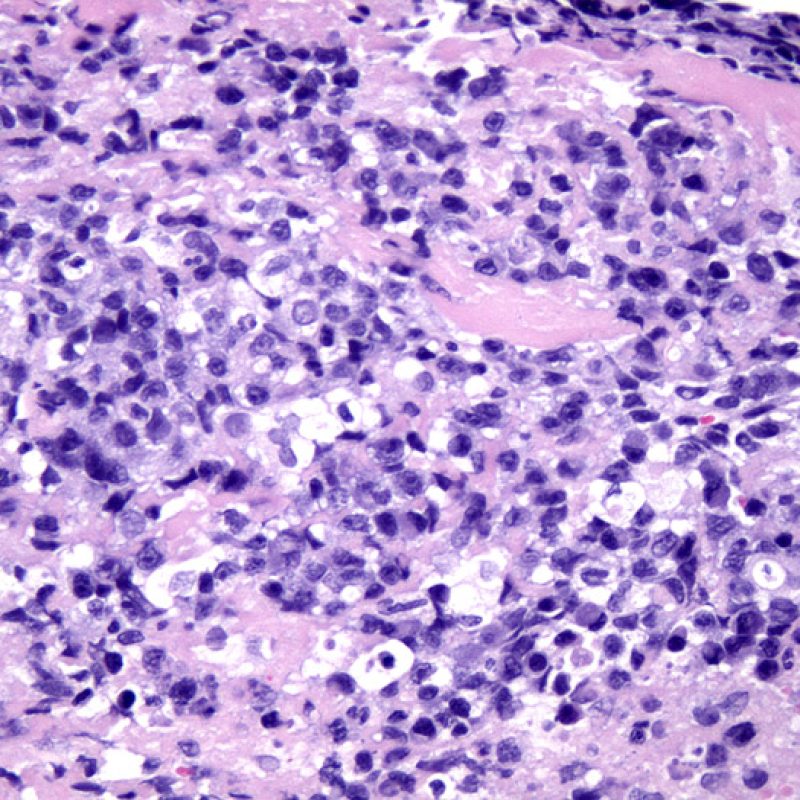
Post transplant lymphoproliferative disorder (PTLD)
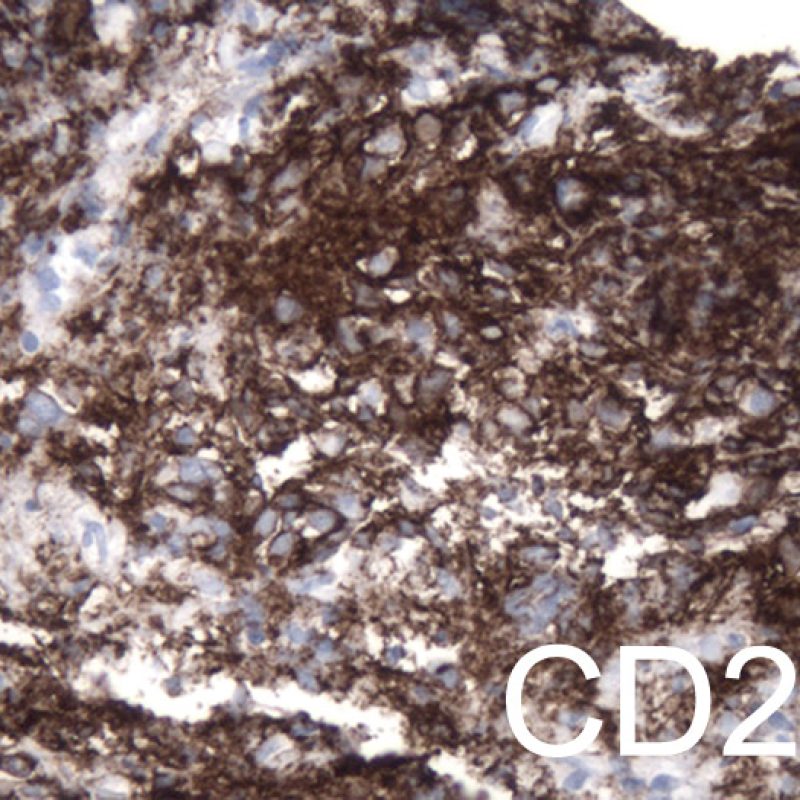
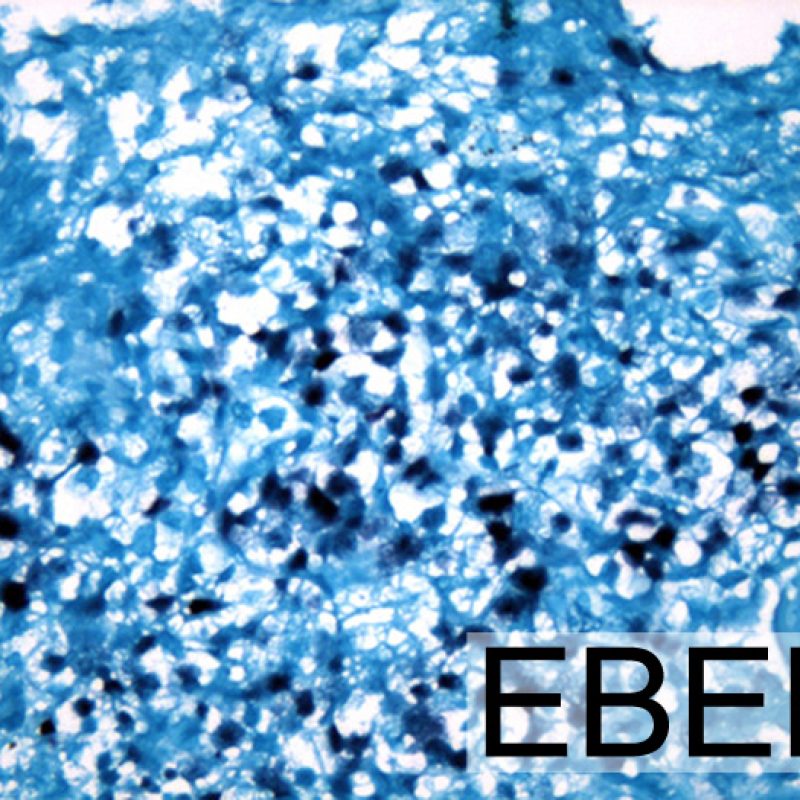
Although post transplant lymphoproliferative disorder (PTLD) occurs in a fairly high percentage (up to 6%) of transplant patients, it is extremely unusual to make that diagnosis on endomyocardial biopsy. Histologically, when it appears, it will typically be sheets of B lymphocytes mimicking acute cellular rejection. These cases are usually EBV positive, so performing EBV in situ hybridization and B cell immunohistochemistry (CD20) in cases suspicious for PTLD is warranted. Also, this diagnosis can sometimes be entertained when a patient fails to respond to what is presumed to be acute cellular rejection. The presence of a significant number of plasma cells in a biopsy is unusual and can be evaluated for a plasmacytoid PTLD. Rarely T cell lymphomas also cause PTLD.
Foreign Body
Small clusters of foreign body giant cells may be seen in endocardium in recipients who have had repeated biopsies. They may contain refractile material, held to be probably fragments of cotton fibre from gauze used to wipe the bioptome between biopsies.
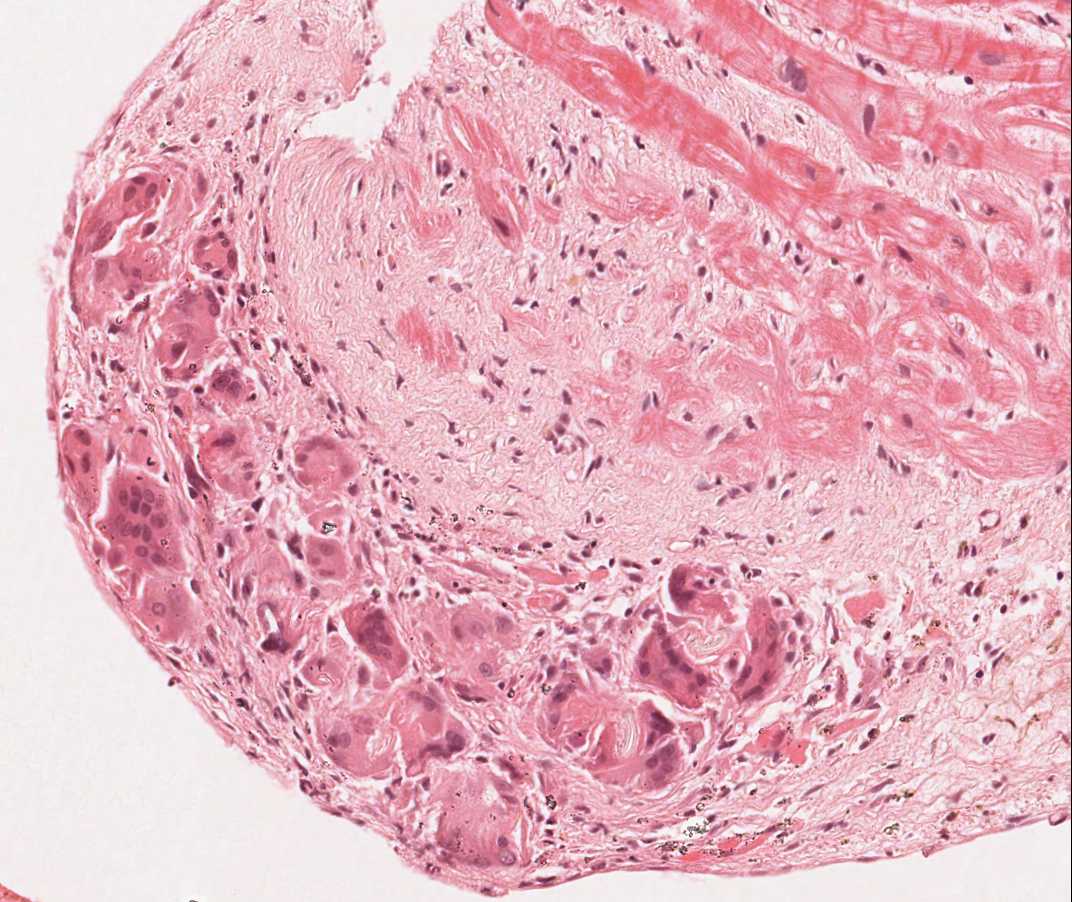
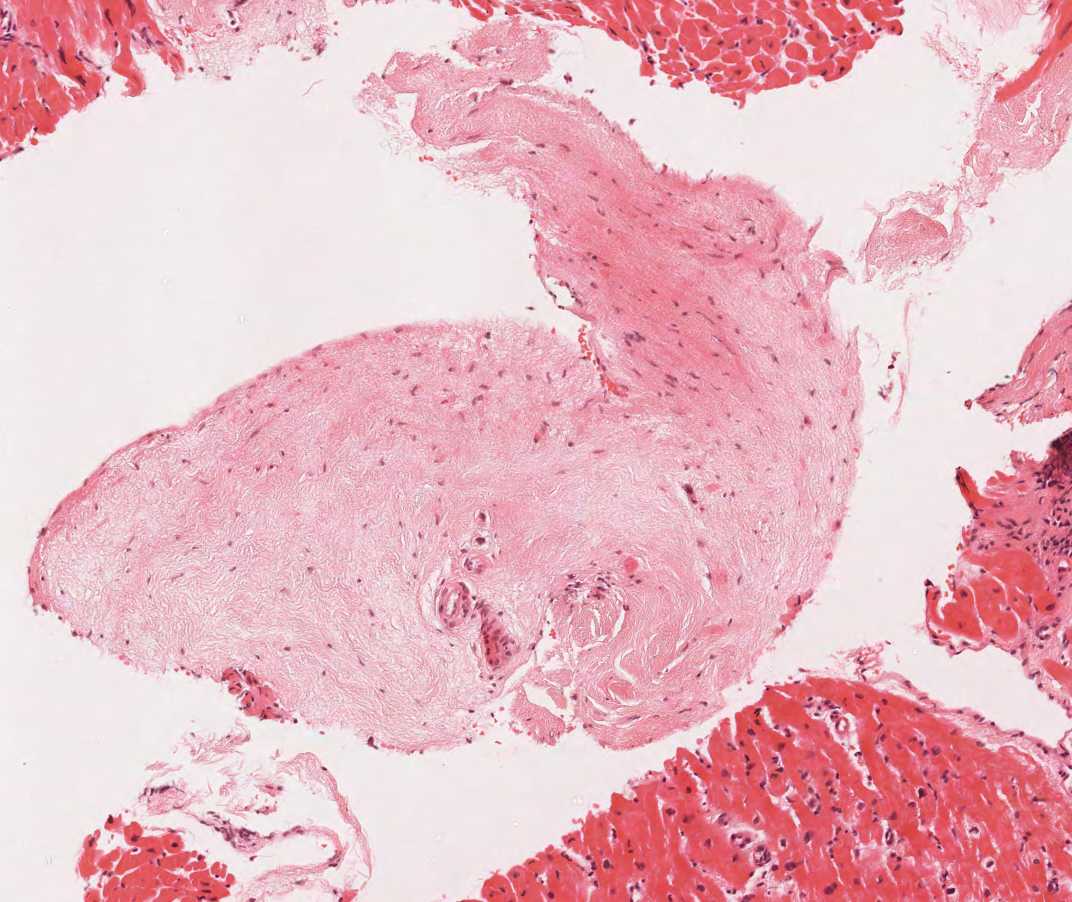
Tricuspid Valve Fragments and Liver Fragments
About 12% of transplant recipients develop a tricuspid murmur in the years post-transplant. The most common cause of post transplant tricuspid regurgitation is congestive heart failure, annular dilatation or pulmonary hypertension. Rarely, this is because of accidental tearing of the valve cusp or snagging of a chorda by the bioptome as it crosses the tricuspid valve. If pieces of either are seen in the biopsy sample the clinician should be informed. Although biopsy of chordal fragments are usually well tolerated, a flail valve due to a big enough defect may affect graft function significantly. A valve or chord fragment will be essentially be a narrow band of fibrous tissue with elastic fibers aligned horizontally with the long axis of the material. Endothelial cells may be seen on both sides, unlike a piece of thickened endocardium, although this distinction can be difficult to make.
Extremely rarely, the bioptome can pass from the superior vena cava into the inferior vena cava, without clinical appreciation, and sampling can be taken from the liver instead of the heart. Thus a piece of liver on a biopsy slide can come from the patient or from a possible contaminant (“tissue pickup”) in the clinical laboratory from poor technique.
Artifacts
A number of artefacts may be seen in endomyocardial biopsies. The more important of these are illustrated below. These artifacts may be misinterpreted by the unwary as being significant pathologies even though they are not.
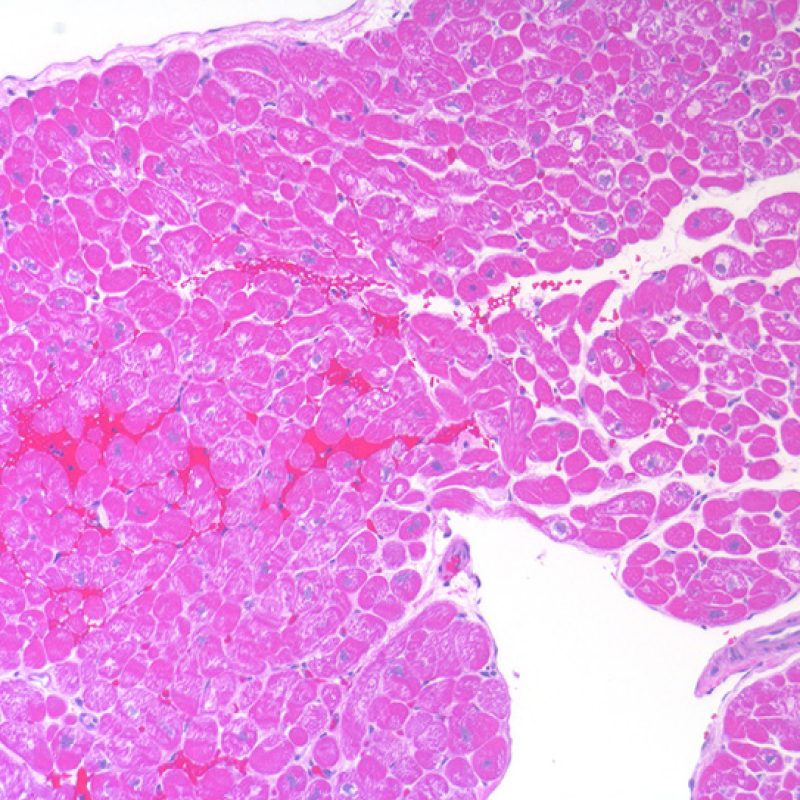
Contraction Bands
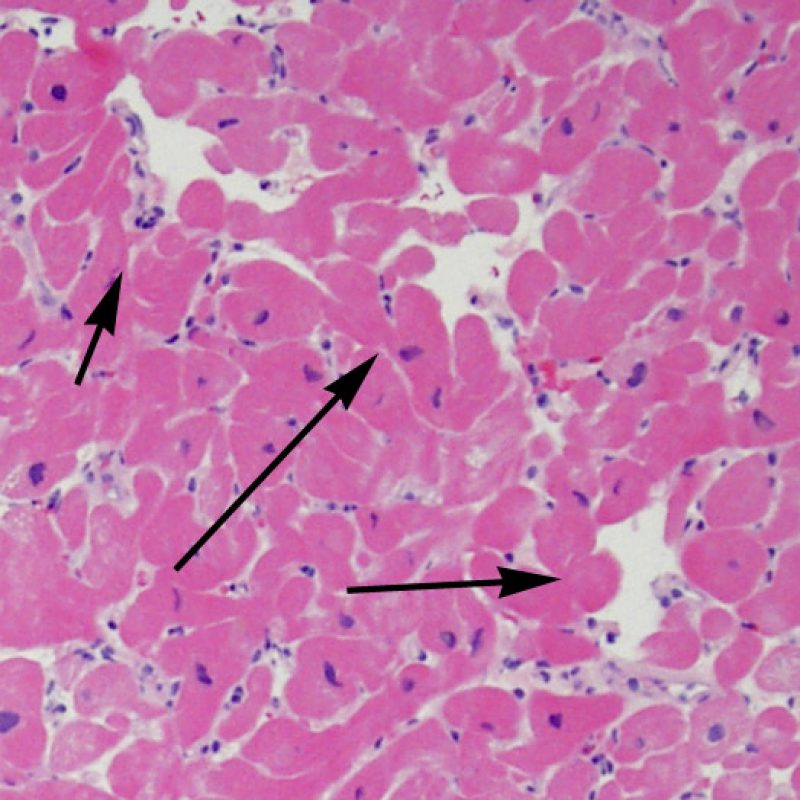
When endomyocardial biopsies are taken and placed into formalin they must be handled gently to prevent crush artefact occurring. But however gently they are treated, myocyte retraction is inevitable because the biopsy is, by definition, at a cut edge of myocardium. The banding is worsened if the fixative is chilled rather than at room temperature. The Z bands become thickened and the sarcomeres become hypercontracted but myofibrils remain intact. The result is contraction banding. This is commonly observed and is often extensive, but is not of pathologic significance.
Contraction banding must be differentiated from contraction band necrosis/ myocytolysis, seen in post-operative/post-intervention reperfusion injury, in patients on high inotrope therapy in a wide range of clinical circumstances and in the hearts of donors who have died from intra-cranial haemorrhage This form of necrosis affects single cells or small groups of cells surrounded by normal-appearing myocardium. The Z bands linking individual myocytes are broken up. Sarcomeric myofibrils fragment and take on a granular basophilic hue often because of mineralization due to intracellular deposition of calcium.
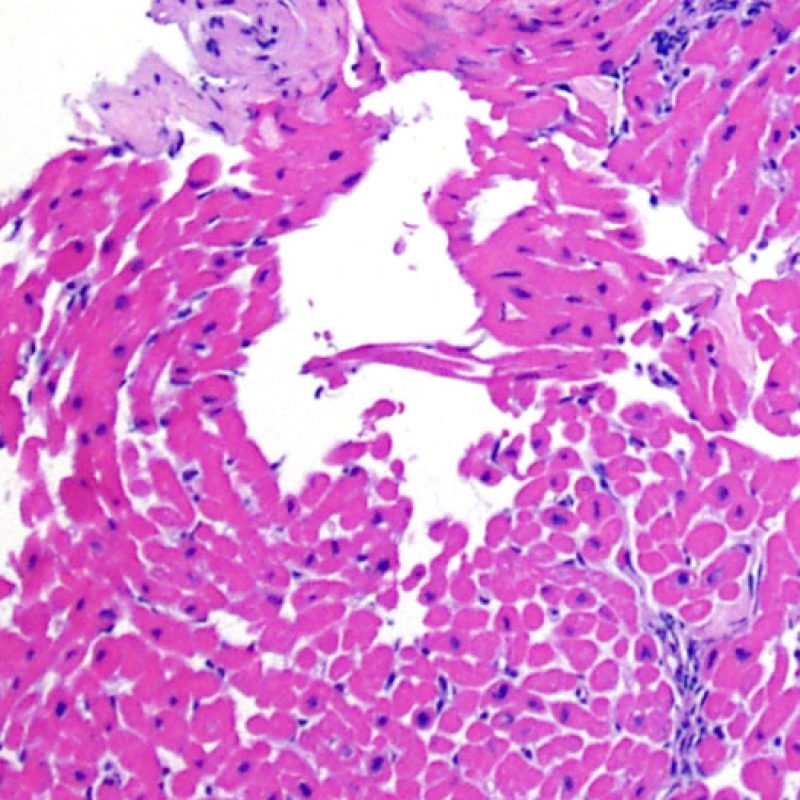
Telescoping of blood vessels
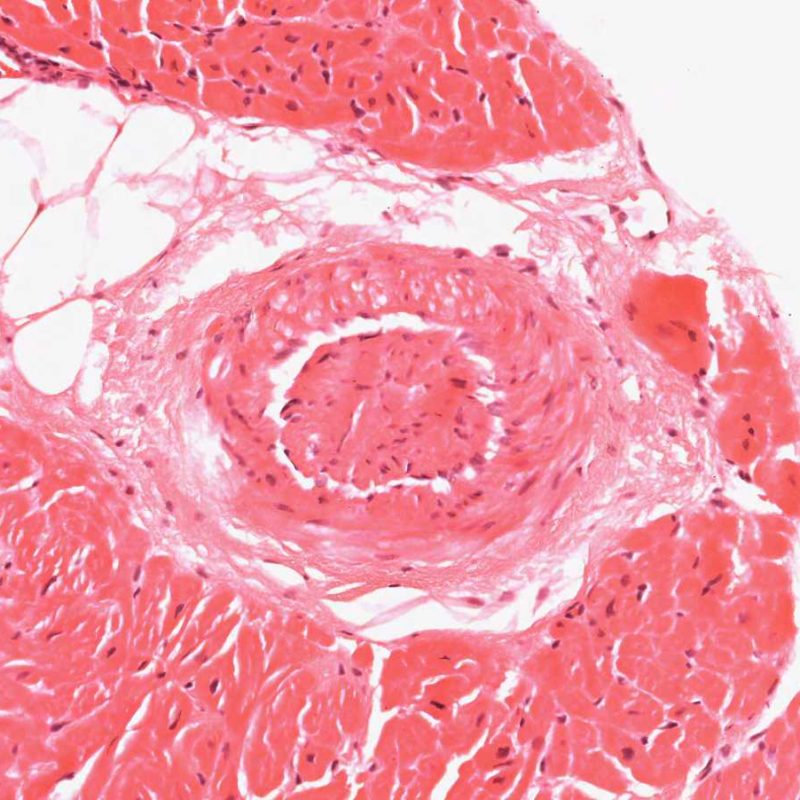
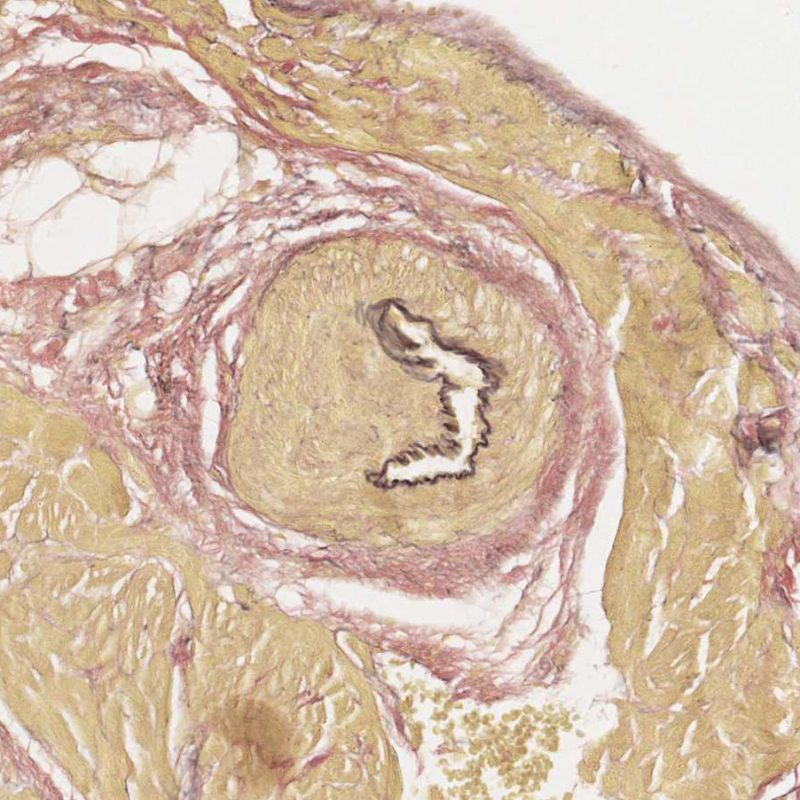
A common artefact intrinsic to the biopsy tissue is “telescoping” of arteriolar vessels due to the elastic recoil of the media that occurs when the bioptome cuts and pulls the biopsy from the septum. The vessel recoils into itself and the resulting appearance can mislead the unwary to misinterpret it as small-vessel cardiac allograft vasculopathy (CAV) as the lumen is inevitably occluded in the process. However, close inspection, aided by a connective tissue stain, such as Miller’s Elastic Van Gieson stain, shows a normal intima and clearly outlines the internal and external elastic lamina of the vessel wall.
Telescoping is of no clinical significance but simply reflects the site of the biopsy – close to the interventricular septum where small penetrating branches of left anterior descending coronary artery may be inadvertently biopsied. However CAV may be diagnosed very occasionally by this route so close checking of vessel architecture is recommended!
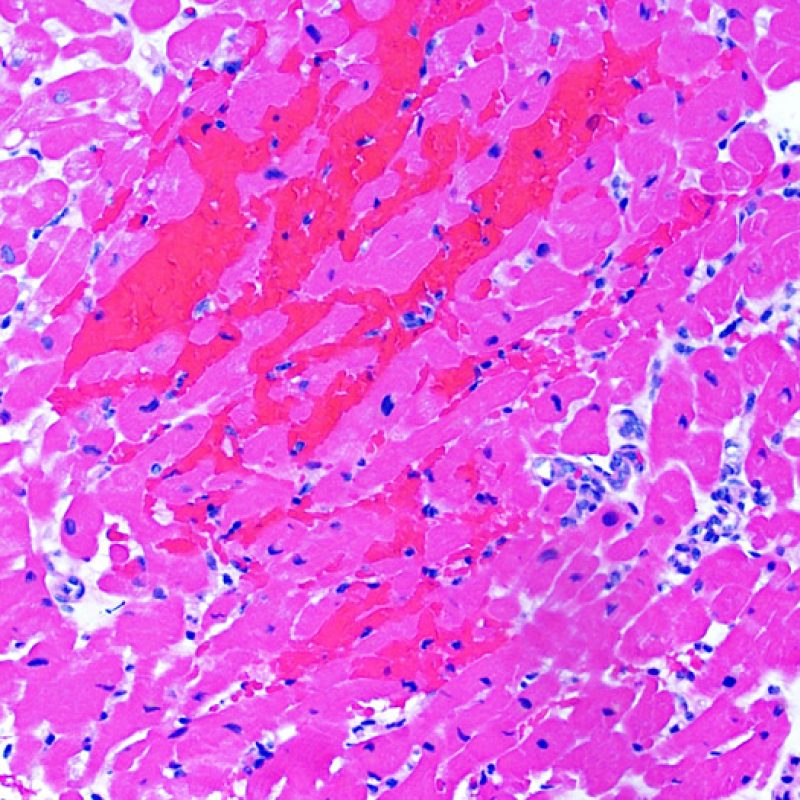
Biopsy-induced hemorrhage
Occasionally the procurement of biopsy pieces can cause tissue hemorrhage. Unlike the hemorrhage seen in severe rejection (Grade 3R), this hemorrhage typically is not associated with marked inflammation or tissue edema.
Pediatric Biopsy Considerations
Pediatric biopsies for cardiac transplant are generally seen in pediatric hospitals or in institutions with joint adult and pediatric programs. Routine EMB for pediatric follow up is less frequent than adult transplants. Although all of the same information applies to pediatric cases as was described for adult cases, there are a few special features to be aware of that are covered here.
Biopsy size
Particularly for infant transplantions, the size of biopsy pieces can be significantly smaller in the pediatric population. This is a result of using smaller bioptomes due to narrower vascular access and the small size of the heart. Pediatric cases should still have a minimum of 3 pieces of tissue for evaluation.
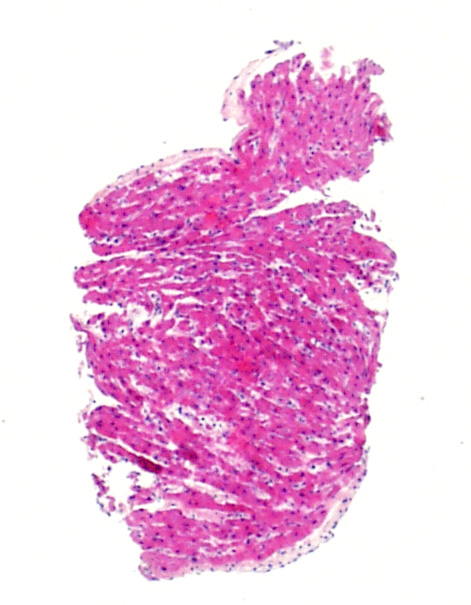
Biopsy size
Myocytes of neonatal and pediatric hearts are significantly smaller than adolescent and adult myocytes. Thus, the overall nuclear to cytoplasmic ratio is such that the biopsies look significantly more cellular. If you are used to evaluating adult biopsies, you will want to keep this in mind and look specifically for lymphocytes, not just a higher N/C ratio. Also, pediatric endothelial cells can appear plumper and will stand out more on biopsy. Again, this is something to be aware of in this age population.
Rates of Rejection
In one of our institutions we observed that rates of rejection in the pediatric population (age 1-18) were roughly double that of the adult population. One of the biggest problems was medication compliance among teenage transplant patients. A second potential cause is more robust immune systems in children than in the elderly. If you find you are seeing more rejection among your pediatric population than an adult population, that is reasonable.
About
This web tutorial would not have been possible without the support of the SCVP and AECVP and the work of the individuals listed below.
Content Creators
- Md. Shahrier Amin – West Virginia University
- Jim Atkinson – Vanderbilt University
- Margaret Burke – Harefield Hospital (Retired)
- Jay Fallon – East Carolina Univeristy
- Marc Halushka – Cleveland Clinic
- Debra Kearney – Texas Children’s Hospital (Retired)
- Joseph Maleszewski – Mayo Clinic
- Richard Mitchell – Brigham and Women’s Hospital
- He Wang – Yale University
Editorial Staff / Tutorial Videos / Web Programming
- Desley Neil – University Hospitals Birmingham
- Gayle Winters – Brigham and Women’s Hospital (Retired)
- John Veinot – Ottawa Hospital (Retired)
- Richard Mitchell – Brigham and Women’s Hospital
- Toby Cornish – University of Colorado
- Marc Halushka – Cleveland Clinic
Additional Images
- Robert Padera – Brigham and Women’s Hospital
- William D. Edwards -Mayo Clinic (Retired)
Translations
- He Wang – Yale University (Chinese)
- Saeko Yoshizawa – Columbia University (Japanese)
- Olga Nikolskaia – Johns Hopkins University (Russian)
- Lubov A. Batoroeva (and family) – Irkutsk Medical Institute (Russian)
- Ornella Leone – -Poloclinico S.Orsola-Malpighi (Italian)
- Claire Toquet – University Hospital Nantes (French)
- Jean-Paul Duong Van Huyen and Patrick Bruneval – HEGP Paris (French)
- Rosa Riberta and Joaquim Majó – RVI, Newcastle Upon Tyne, UK (Español)
- Silvio H. Litovsky – University of Alabama at Birmingham (Español)
Was this helpful to you? Please consider donating to the SCVP Educational Endowment Fund to pay for our educational initiatives. We hope to make additional tutorials on a variety of cardiovascular topics.